& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
- Suzuki coupling: A most common method to synthesize biaryls, conjugated arenes and styrenes, involving C-C bond formation

- Reactant-1 (Organoboron compound; Nucleophile): Aryl/Alkenyl/Allyl/Alkyl borane/boronic acid/boronic esters (boronate)
- Reactant-2 (Organic Electrophile): Organohalide/triflate
- Solvents: THF, DMF, Dioxane, toluene
- Palladium catalyst: Pd catalyst in zero oxidation state [Pd(0)]. Both Pd(0) and Pd(II) sources can be used, though the active species is Pd(0)
(a) Catalysts: Pd(PPh3)4 (tetrakis), (Ph3P)2PdCl2 (dikis) etc. – Pd(0) sources
(b) in-situ generation of catalysts: Pd (II) sources [PdCl2, Pd(OAc)2, Pd2(dba)3, etc] and a ligand [Phosphine etc.]
- Bases: Inorganic bases (NaOH, Na2CO3, K3PO4, Ba(OH)2, NaF/K etc.), Organic bases (DIPEA, Et3N et.)
- Possible side reactions: (a) Proto-deboronation (b) Dehalogenation and (c) Homocoupling of boronic acid

- Organoboron compounds are generally air and water stable and the products are non-toxic (borax)
- Organotrifluoroborates are an alternative to boronic acids, boronate esters, and organoboranes
Useful Links on Reagent & Reaction:
- Suzuki Coupling (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
- Suzuki Coupling (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
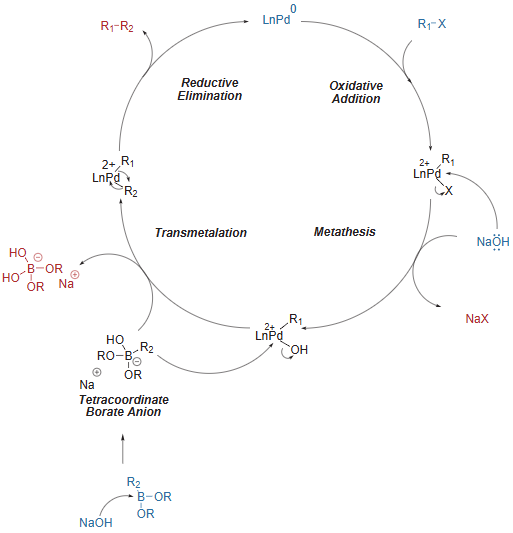
Suzuki Coupling – General Mechanism
Pd enters the catalytic cycle only as Pd(0) with 2 ligands
The mechanism involves four key steps;
(a) Oxidative addition: Oxidative addition of the organohalide to the Pd(0) catalyst, creating a halide-Pd(II)-alkyl complex
(ii) Metathesis (Ligand exchange): Replacement of the halide attached to the complex with the anion of the base
(iii) Transmetallation: The organoboron compound and base form a tetracoordinate borate anion, which increases the nucleophilicity of its alkyl/aryl group to exchange with the attached anion of the base, forming a Pd(II) complex with two alkyl groups
(iv) Reductive elimination: Reductive elimination facilitates the formation of a single bond between the alkyl/aryl groups, regenerating the Pd(0) catalyst.
After reductive elimination, the active Pd(0) species is regenerated and re-enters the catalytic cycle
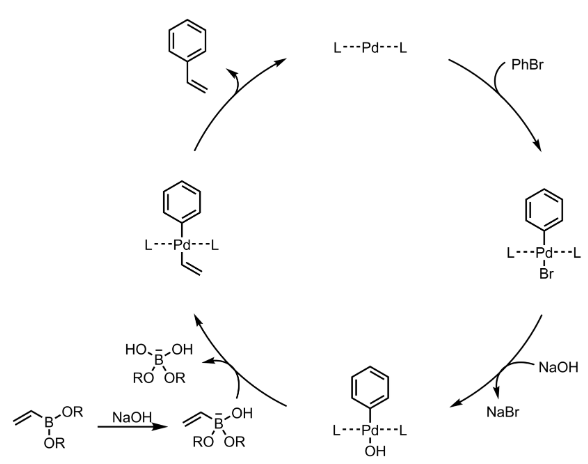
Suzuki Coupling – Mechanism with specific example
Additional details

General Procedure-1 :
Bromo-aromatic (1 equiv.), phenyl boronic acid (1.5 equiv.), PHOS ligand (0.1 equiv.), and tris(dibenzylideneacetone)dipalladium chloroform complex (0.05 equiv.), and CsF (10 equiv.) were mixed into THF. The mixture was stirred at rt. for 12 hours. The resulting mixture was filtered with Celite, and the filtrate was separated by silica gel column to afford the desired product.
General Procedure-2 (biphasic condition) :
Halo-aromatic ring (1 equiv.), phenyl boronic acid (1.2 equiv.), PdCl2(dppf) (0.1 equiv.), and 2 M Na2CO3 (10 mL) were mixed with toluene/dioxane (4:1, 10 mL). The mixture was degassed, and stirred for 4 hours at 85 oC with nitroegn atmosphere. The resulting mixture was filtered with celite, and the filtrate was separated. The organic layer was concentrated. The resulting residue was separated by silica gel column.
General Procedure-3 (Microwave condition; for lab-scale only) :
The halo-aromatic ring (1 equiv.), boronic acid (1.5 equiv.), PdCl2(dppf) (0.1 equiv.), and 2 M K2CO3 (10 equiv.) were dissolved into N-dimethyl acetate amide. The mixture was reacted on a microwave reactor at 150 oC for 20 min. The resulting mixture was filtered, and the filtrate was purified by column chromatography.
In Microwave condition, ensure that no solid is present. Also, under this condition, even less active chloro substituent could be used.
Note:
- Suzuki coupling is most commonly used to form C-C bond, resulting in biaryls, conjugated alkenes and styrenes.
- The reaction involves mild conditions and easy work-up
- The coupling reaction is highly stereospecific and regioselective
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
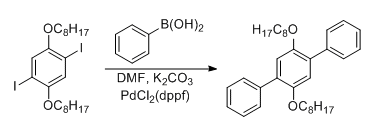
- Suzuki coupling of 1,4-dioctyloxy-2,5-diiodobenzene and phenylboronic acid (ChemSpider) — Open access
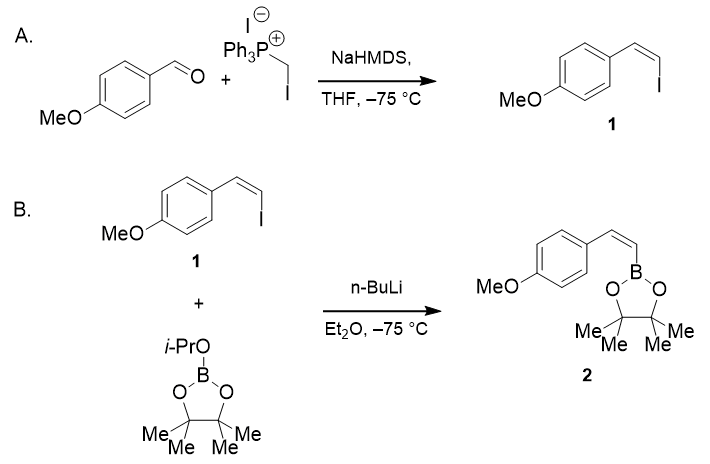
- Anhydrous, Homogeneous, Suzuki-Miyaura Cross-Coupling of Boronic Esters using Potassium Trimethylsilanolate (OrgSyn) — Open access
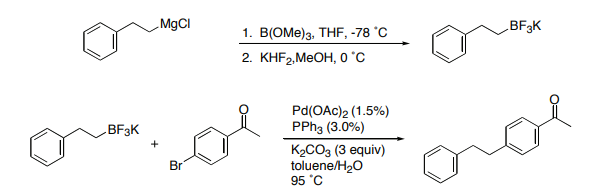
- 1-(4-ACETYLPHENYL)-2-PHENYLETHANE from POTASSIUM 2-PHENETHYLTRIFLUOROBORATE and 4-BROMOACETOPHENONE (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
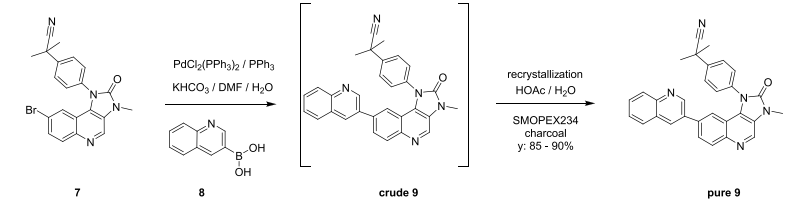
WO2007121484, page no.145

WO2010038081, page no. 110
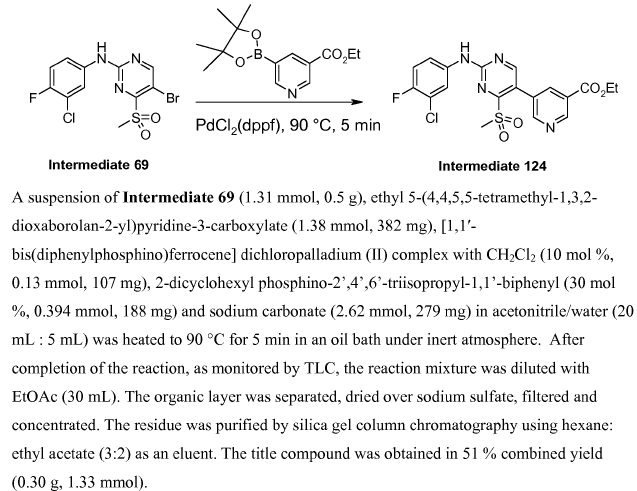
WO201003808, page no. 123
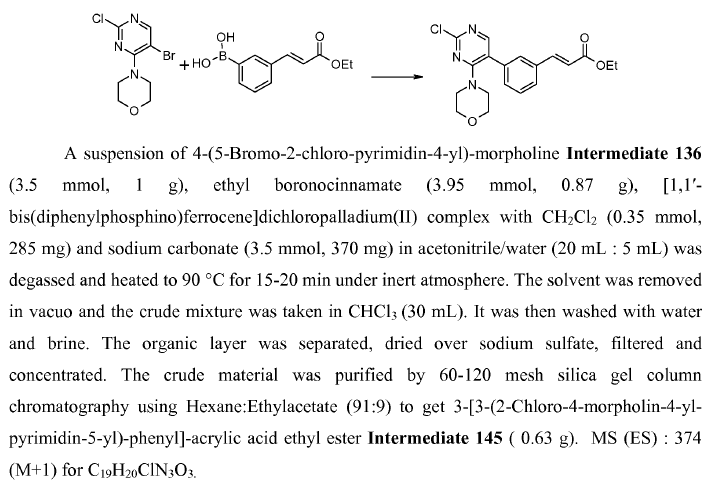
WO2010045258, page no. 126
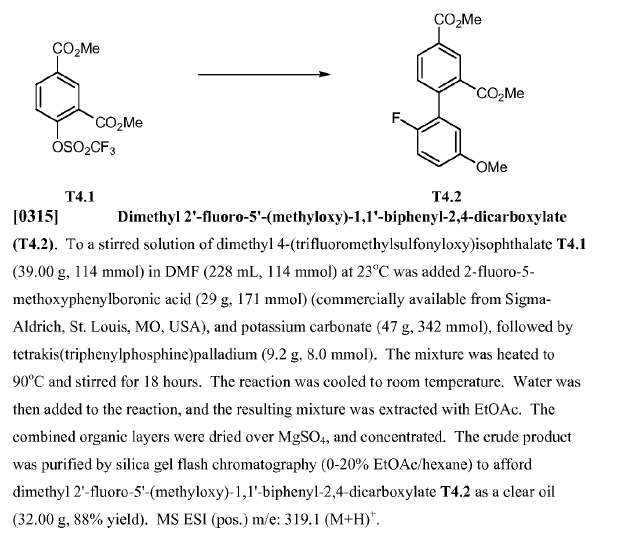
WO2010045258, page no. 142

WO2007117607,page no. 325
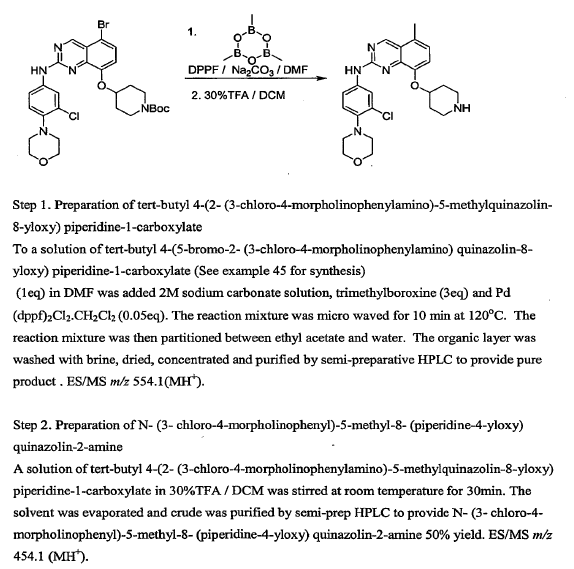
WO2010045258, page no. 154

Green Chem
Suzuki coupling is the most common cross-coupling reaction that has been carried out on large-scale.
Scale-Up Typical Procedure:
- Development of a Robust Synthesis of Dactolisib on a Commercial Manufacturing Scale (OPRD, 2017) – 155 Kg batch (Bromo derivative), Boronic acid (70 Kg) and Pd cat. (0.78 Kg) are used
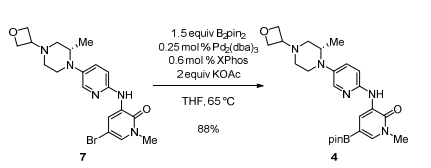
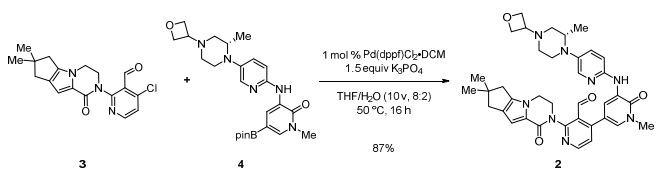
- Development of an Efficient Manufacturing Process for Reversible Bruton’s Tyrosine Kinase Inhibitor GDC-0853 (OPRD, 2018) – (a) BPin syn: 120 Kg batch (Br compd) and B2pin2 (105 Kg) are used; (b) Suzuki coupling: 74 Kg (Cl compd) and Bpin compd (122 Kg), Pd cat. (1.74 Kg Kg) are used.
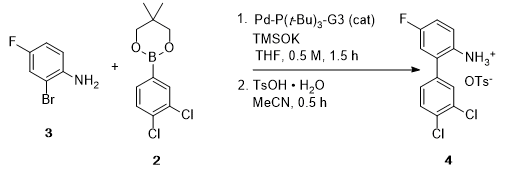
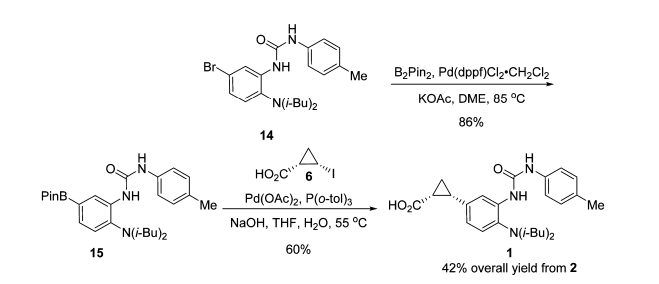
- Development of a Scalable Synthesis of BMS-978587 Featuring a Stereospecific Suzuki Coupling of a Cyclopropane Carboxylic Acid (OPRD, 2018) – BPin syn (1.25 Kg batch) and Suzuki coupling (400 g) are reported
Green Chemistry Aspects:
Useful articles for Scale-up:
- Identification and Elimination of an Unexpected Catalyst Poison in Suzuki Coupling (OPRD, 2018, 22, 111-116) – A useful information for every scientists who performs Suzuki reaction in the manufacturing plant
Suzuki Coupling – Reviews :
- Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111 (3), 2177–2250
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005, 127 (13), 4685–4696.