& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
- Stille coupling: C-C bond formation involving organostannane and organohalide

- Reactant-1 (Organoboron compound; Nucleophile): Aryl/Alkenyl/allyl tin compounds (Stannanes)
- Reactant-2 (Organic Electrophile): Organohalide/triflate
- Solvents: THF, DMF, Dioxane, toluene
- Palladium catalyst: Pd catalyst in zero oxidation state [Pd(0)]. Both Pd(0) and Pd(II) sources can be used, though the active species is Pd(0)
(a) Catalysts: Pd(PPh3)4 (tetrakis), (Ph3P)2PdCl2 (dikis) etc. – Pd(0) sources
(b) in-situ generation of catalysts: Pd (II) sources [PdCl2, Pd(OAc)2, Pd2(dba)3, etc] and a ligand [Phosphine etc.]
- Possible side reactions: Oxidative Homocoupling of organostannanes
- Unlike other organometallic compounds, organostannanes are not sensitive to moisture or air. Hence, handling these compounds are easy.
Useful Links on Reagent & Reaction:
- Stille Coupling (SynArchive) – Excellent compilation of reaction schemes with references
Mechanism
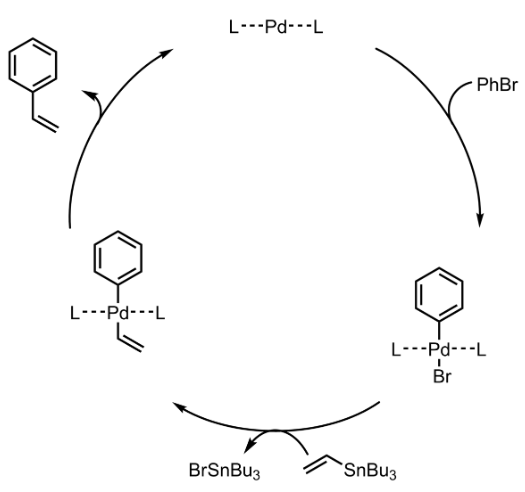
Stille Coupling – General Mechanism
- The mechanism follows Oxidative addition – Reductive elimination cycle.

Stille Coupling – Mechanism with specific example
Additional details

General Procedure-1:
Halo-aromatic ring (1 eq.), tributyl (1-ethoxyvinyl) tin (1.2 eq.), Pd(PPh3)4 (0.05 eq.), and K2CO3 (1.2 eq.) are mixed in toluene (10 Vol). The mixture is degassed and heated to 100 oC for 2 hours under nitrogen atmosphere. The mixture is concentrated and the residue is mixed with 2N HCl. The resultant aqueous mixture is stirred for 2 hours and extracted with ethyl acetate. The organic layer is dried, concentrated, and the residue is separated by silica gel column chromatography to get the desired compound.
General Procedure-2:
A mixture of bromo-aromatic compound (1 eq.), Me4Sn (10 equiv.), Pd(OAc)2 (0.05 equiv.), and PPh3 in DMF is heated to 100 oC for 30 min. The reaction is monitored by TLC. The resulting mixture is diluted with ethyl acetate, and washed with brine. The organic layer is dried and concentrated. The residue is purified by silica gel column chromatography.
Work-up tips:
- Bu3SnX by-products can be removed by filtering through silica made up with ~2-5% triethylamine in the eluent or by washing the reaction mixture with a saturated aqueous solution of KF.
Note:
- Stille coupling is very similar to Suzuki coupling.
- As organotin compounds are air-stable, commercially available or easily synthesized, it is very popular. However, significant toxicity of organotin compounds and the difficulty to remove traces of tin-byproducts makes less preferable, as compared to Suzuki
- This reaction is highly stereospecific and regioselective, as similar to Suzuki.
- Reaction conditions are compatible with carboxylic acid, amide, ester, nitro, ethers, amine, hydroxyl, ketone, and formyl groups.
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
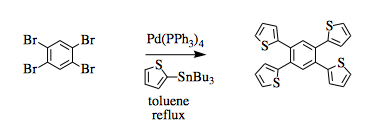
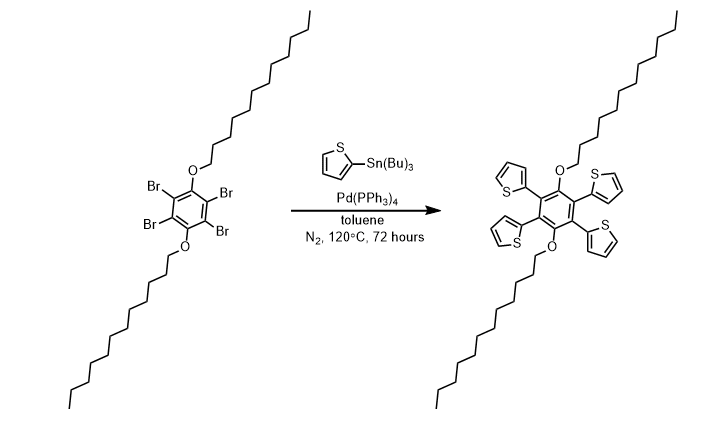
- Stille coupling of thiophene to 1,2,4,5-tetrabromo-3,6-bis(dodecyloxy)benzene (ChemSpider) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
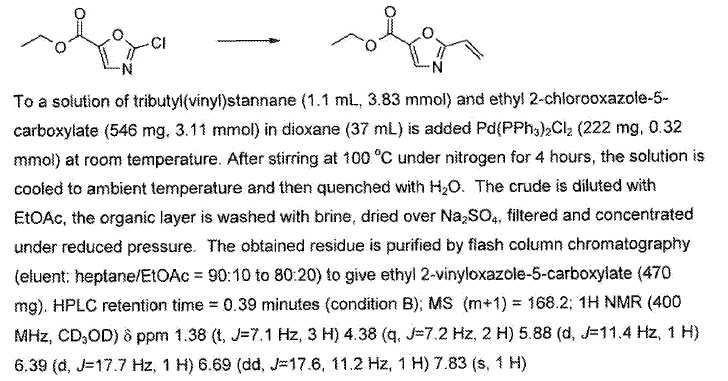
WO2010136493, page no. 235
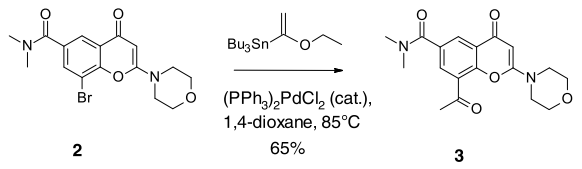
WO2007121484, page no. 136

Green Chem
- Significant toxicity of organotin compounds poses major challenges in manufacturing
- Tin reagents exhibit poor solubility in water and hence excess reagents and its by-products could not be removed during aqueous work-up. This makes it less preferable, as compared to Suzuki
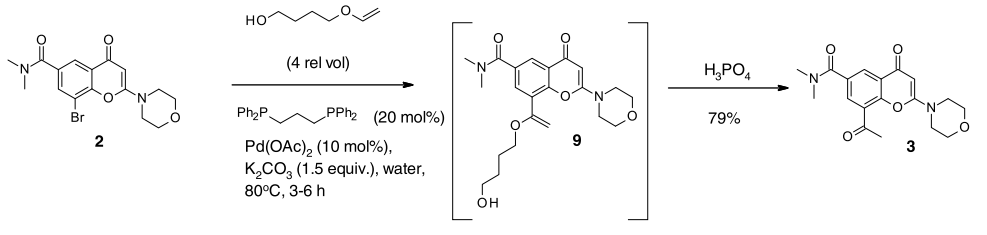
In the following article, Stille coupling that was used in Medicinal chemistry route has been replaced by Heck coupling for Scale-up
- Med. Chem Route involving Stille coupling
- Manufacturing Route involving Heck coupling