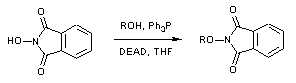& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
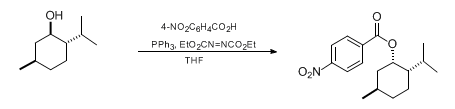
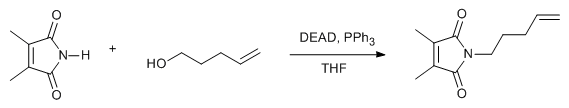
- Mitsunobu reaction is very useful in forming C-O, C-N, C-S and C-C bond formation
- It is the reaction between alcohol and different nucleophiles using PPh3 and azodicarbozxylate (DIAD or DEAD). The reaction proceeds with inversion of configuration (SN2)

- Reactant-1 (Alcohol): 1o and 2o alcohols
- Reactant-2 (Nucleophile): Phenol, thiol, amine, azide, phthalimide (Nucleophile whose pKa ≤ 15)
- Reagents: PPh3 (or) P(n-Bu)3, dialkyl azodicarboxylate (DEAD or DIAD)
- Solvents: THF, DCM, Dioxane, toluene
- Product: Substitution product containing C-O, C-S, C-N and C-C bonds
- by-products: Triphenylphosphine oxide (TPPO), hydrazine dicarboxylate
- Reaction type: SN2
- The by-products formed are triphenylphosphine oxide (by oxidation of triphenylphosphine) and hydrazine dicarboxylate (by reduction of DEAD or DIAD)
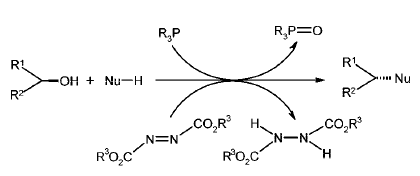
- Nucleophile (Nu-H) should be acidic enough to protonate azodicarboxylate (refer to mechanism)
| Reactant | Nucleophile | Product |
|---|---|---|
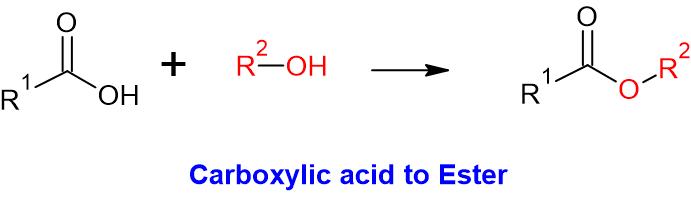
| Alcohol | Carboxylic acid | Ester |
| Alcohol | Alcohol or Phenol | Ether |
| Alcohol | Thiol | Thioether |
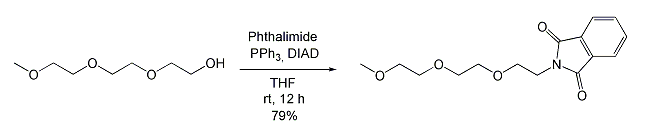
| Alcohol | Phthalimide | N-alkylation (on cleavage, it forms C-N bond) |
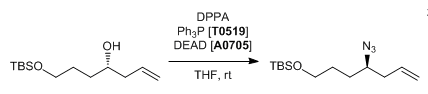
| Alcohol | DPPA | Azide |
| Alcohol | Cyanohydrin (HCN source) | Nitrile (C-CN bond) |
| Alcohol | EWG-CH2-EWG (active methylene compds) | C-C bond formation |
- Also, refer to other reactions involving triphenyl phosphine: Appel reaction, Suzuki Coupling
Useful Links on Reagent & Reaction:
- Mitsunobu reaction (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
- Mitsunobu reaction (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
Mitsunobu reaction – Mechanism
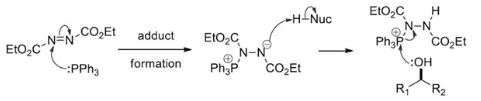
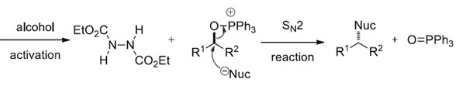
It involves (a) Formation of Betaine intermediate (zwitterionic adduct), when PPh3 attacks DEAD or DIAD (b) Transfer of proton from nucleophile to adduct to form phosphonium ion (c) Formation of oxyphosphonium ion, by expelling hydrazine dicarboxylate (d) Nucleophilic attack in SN2 fashion
Additional details

General Procedure:
To a solution of alcohol (1 eq.) in THF (10 Vol) is added triphenyl phosphine (TPP, 1.5 eq.), and NUCLEOPHILE. The mixture is cooled to 0 oC. DIAD (1.5 eq) is then added dropwise at 0 oC, and stirred at room temperature for 6 to 8 h. The reaction is monitored by TLC. (The formation of triphenylphosphine oxide, TPPO, as a solid is the indication of the progress of the reaction).
The reaction mixture is diluted with EtOAc or DCM (15 Vol) and filtered to remove the by-product triphenylphosphine oxide. The filtrate (i.e. organic layer) is successively washed with water (15 ml x 2), aq. NaHCO3 solution (to remove unreacted acid) and brine solution (15 ml), dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography.
- NUCLEOPHILE shall be alcohol, DPPA, carboxylic acid, Thiol, Phenol, Phthalimide. The key criteria of nucleophile (Nu-H) is that it should be acidic enough (pKa ≤ 15; refer to mechanism to understand more)
Note:
- 1o and 2o alcohols are generally used. 3o alcohols do not react
- The most preferable solvent is THF. Other solvents such as DCM and Dioxane could also be used. The reaction is usually performed at RT
- The by-product namely triphenylphosphine oxide (TPPO) is usually removed by filtration
- The order of addition of reagents matters. Alcohol, Nucleophile and PPh3 are dissolved in a suitable solvent and cooled to 0 oC. DIAD or DEAD is then added.
- When DPPA is used as a nucleophile, Mitsunobu reaction results in the formation of organic azides. Organic azides are known to be explosive. Safety studies are to be carried out before performing on large-scale. It is also better not to heat the reaction. It is always better to find an alternate synthetic approach to avoid azides
- DIAD is Diisopropyl azodicarboxylate. It comes as 40% in toluene. Azodicarboxylic esters are susceptible to explosion when subjected to heat, impact and friction. In order to reduce the risk, azodicarboxylic esters are available as a 40% solution in organic solvents.
| Reactant | Nucleophile | Product |
|---|---|---|
| Alcohol | Carboxylic acid | Ester |
| Alcohol | Alcohol or Phenol | Ether |
| Alcohol | Thiol | Thioether |
| Alcohol | Phthalmide | N-alkylation (on cleavage, it forms C-N bond) |
| Alcohol | DPPA | Azide |
| Alcohol | Cyanohydrin (HCN source) | Nitrile (C-CN bond) |
| Alcohol | EWG-CH2-EWG (active methylene compds) | C-C bond formation |
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Acid to Ester under Mitsunobu condition (OrgSyn) — Open access
- Conversion of Polyethylene Glycol (PEG) to N-(3,6,9-Trioxadecyl)phthalimide (Mitsunobu Reaction) (ChemSpider) — Open access
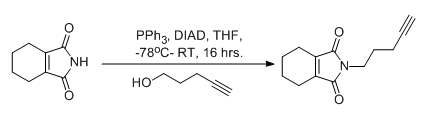
- Alcohol to Azide using DPPA (TCI) — Please click the section ” Applications & Literature” — Open access
- The above reaction (Mitsunobu with DPPA) endangers the potential formation of the toxic and highly explosive hydrazoic acid as well as the stoichiometric formation of an organic azide intermediate. Hence, a detailed safety studies needs to be conducted before performing such reaction.
- It is always desirable to avoid azides and look for alternate methods of making the intermediate

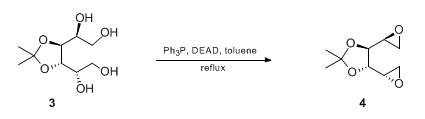
- Diol to Epoxide (OrgSyn) – Intermolecular reaction between two alcohol groups under Mitsunobu — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2007084786, page No. 124
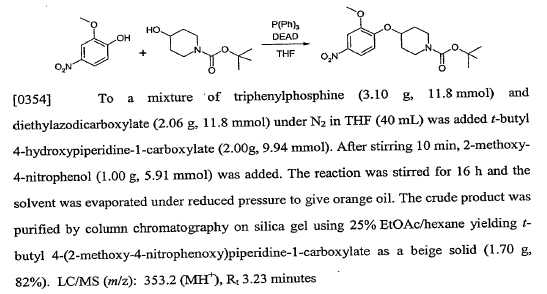
WO2007117607, page No. 315
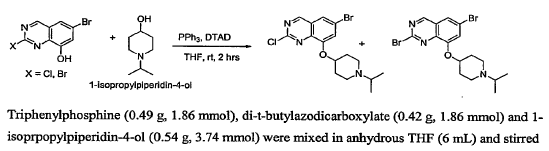

WO2007117607, page No. 325
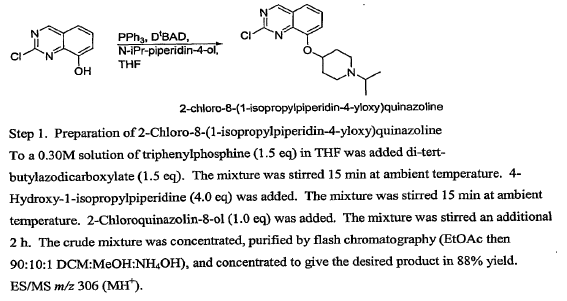
Green Chem
Mitsunobu reaction has been carried out on large-scale and there are several reports available in OPRD
Scale-Up Typical Procedure:
- Development of a Pilot-Plant-Scale Synthesis of an Alkylated Dihydrobenzothiadiazole S,S-Dioxide: Incorporation of a Late-Stage Mitsunobu Reaction (OPRD, 2010) – 5.5 Kg batch; 6.39 Kg triphenylphosphine (TPP) & 4.9 Kg DIAD are used
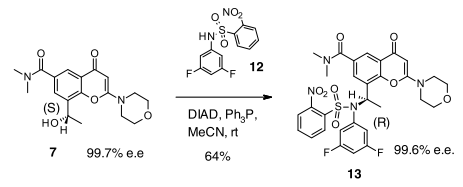
- Mitsunobu Inversion of a Secondary Alcohol with Diphenylphosphoryl azide. Application to the Enantioselective Multikilogram Synthesis of a HCV Polymerase Inhibitor (OPRD, 2011) – 8.44 Kg batch; 6.55 Kg TPP & 5.05 Kg DIAD; 6.87 Kg DPPA are used
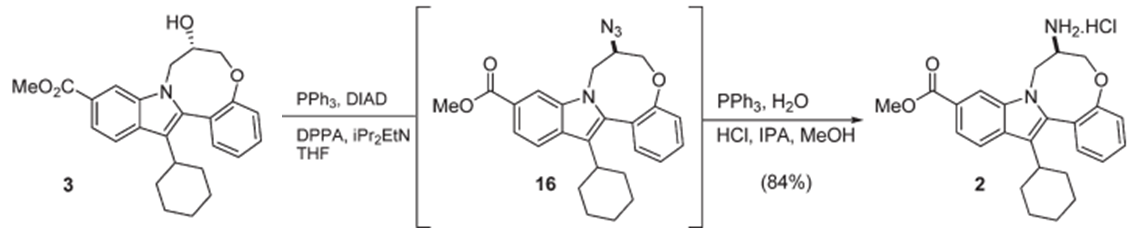
- The above reaction One-pot Mitsunobu inversion and Staudinger reduction
- The above reaction (Mitsunobu with DPPA) endangers the potential formation of the toxic and highly explosive hydrazoic acid as well as the stoichiometric formation of an organic azide intermediate. A detailed safety studies has been conducted and explained in this article
- Development and Scale-Up of an Asymmetric Synthesis of AZD8186 Using the Fukuyama Modification of the Mitsunobu Reaction (OPRD, 2021) – 3.78 Kg batch (sulfonamide); 4.22 Kg TPP & 3.26 Kg DIAD are used
Green Chemistry Aspects:
Useful articles for Scale-up:
Mitsunobu reaction – Reviews :