& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
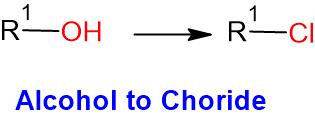
- Thionyl chloride (SOCl2) is commonly used to convert (a) alcohol to Chloride and (b) acid to acid chloride
- SOCl2 is highly reactive. Upon reaction with water and alcohols, it liberates HCl gas violently
- Other reagents that convert alcohols to chloride are oxalyl chloride, (COCl)2, PCl3 and PCl5
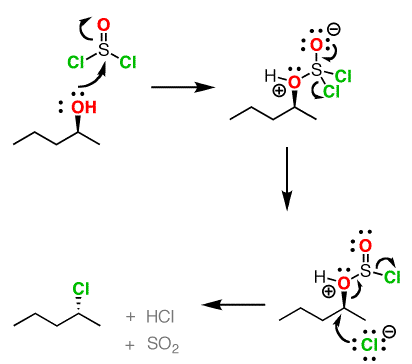
- The reaction occurs through SN2 and hence inversion of configuration (check mechanism below).
- However, addition of pyridine is imporatnt that ensures that the inversion at the stereogenic centter via SN2. When pyridine is not available, the reaction tends to go via SNi (nucleophilic substitution with internal return), resulting in retention in configuration
SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi (Master Organic Chemistry) – A nice explanation is provided here
- The conversion of alcohol to chloride using SOCl2 in presence of tertiary amine (e.g pyridine) is also called “Darzen halogenation“
Advantages
- Inexpensive method on manufacturing scale
- The by-products liberated during the reaction are HCl and SO2, As both being gases, the reaction is usually clean
Disadvantages
- Similar to other sulphur compounds, handling SOCl2 is bit difficult, due to its peculiar unpleasant pungent smell.
- Handling of SOCl2 needs thorough safety studies before planning manufacturing scale
Useful Links on Reagent & Reaction:
- Deoxychlorination (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry information on various chlorinating reagents.
For review papers and other articles,
refer to the tab "References"
Mechanism
Mechanism
Additional details
General Procedure:
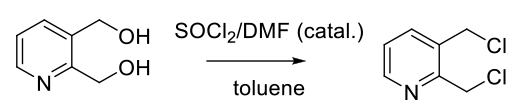
To a solution of alcohol (1 eq.) in toluene (15 Vol) is added thionyl chloride (1.2 eq.) and one drop of dry DMF at 0 oC and stirred at room temperature for 6 h. (If the reaction does not proceed, the reaction mixture shall be heated to 80 oC gradually. However, exothermic nature of the needs to be considered and controlled). The reaction is monitored by TLC.
After the completion of the reaction, excess thionyl chloride is distilled off and the residue is dissolved in DCM. The organic layer is then successively washed with water (10 Vol x 2), aq. NaHCO3 solution and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography
Note:
- The preferable solvents are toluene, DCM
- The by-products liberated during the reaction are HCl and SO2, As both being gases, the reaction is usually clean.
- Catalytic amounts of DMF is added to facilitate the reaction
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
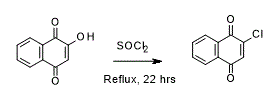
- Chlorination of 2-Hydroxy-1,4-napthoquinone (ChemSpider) — Open access
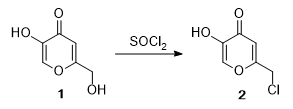
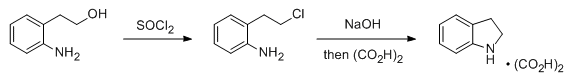
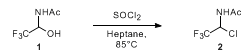
- Chlorination of an alcohol of SOCl2-1 (OrgSyn) — Open access
- Chlorination of an alcohol of SOCl2-2 (OrgSyn) — Open access
- Chlorination of an alcohol of SOCl2-3 (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2010045258, page No. 191
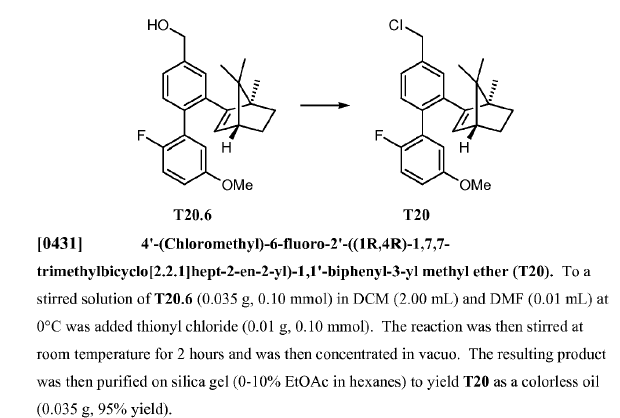
WO2010045258, page No. 203
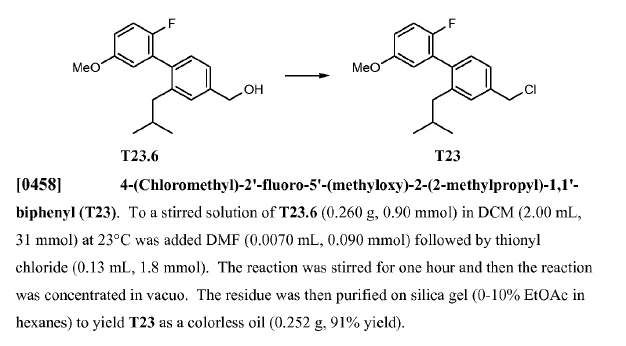
Green Chem.
Thionyl chloride could be carried out on large-scale. However, The by-products liberated during the reaction are HCl and SO2, Appropriate safety controls are to be ensured while performing manufacturing.
Scale-Up Typical Procedure:
- Reaction Safety: A Critical Parameter in the Development of a Scaleable Synthesis of 2,3-Bis-chloromethylpyridine Hydrochloride (OPRD, 2002) – 972 g batch; 940 mL of thionyl chloride is used
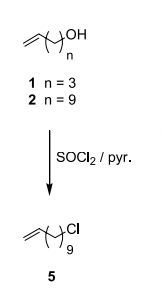
- The facile preparation of alkenyl metathesis synthons (Tetrahedron, 2004) – 200 g batch; 259 g thionyl chloride is used
Green Chemistry Aspects:
- Deoxychlorination (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry information on various chlorinating reagents.
Conversion of -OH to -Cl using SOCl2– References: