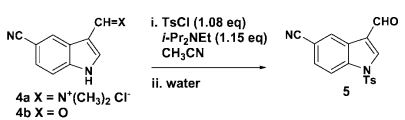
& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
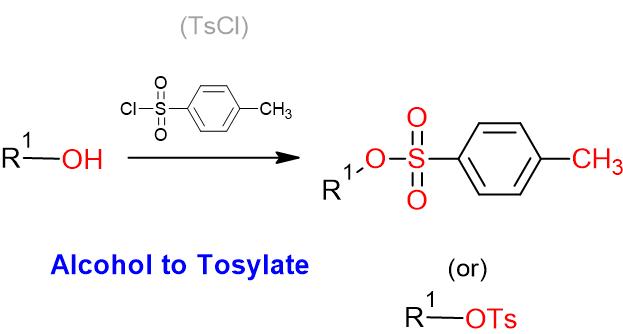
- OH– ion (hydroxide ion), as such, is a poor leaving group, as it is quite strong base. However, OH group shall be converted to a better leaving group such as OMs, OTs or OTf groups for further useful organic conversion
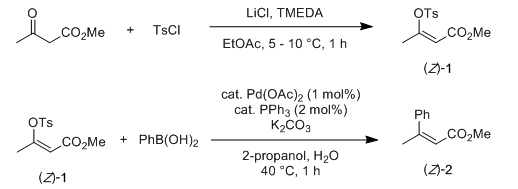
Sulfonates as better leaving groups
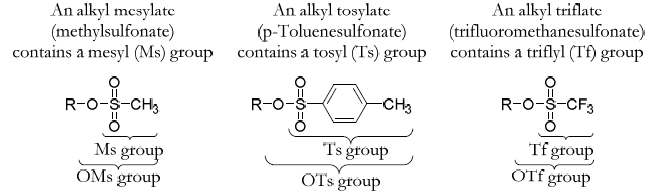
- Leaving group ability; OTf > OTs > OMs
Other ways of synthesizing better leaving groups for SN2 reactions:
Stereochemical outcome of sulfonate ester (OMs, OTs and OTf):
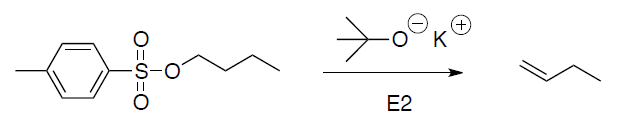
- Alkyl sulfonates undergo SN2 and E2 reaction in a manner identical to that of alkyl halides
(a) Substitution:
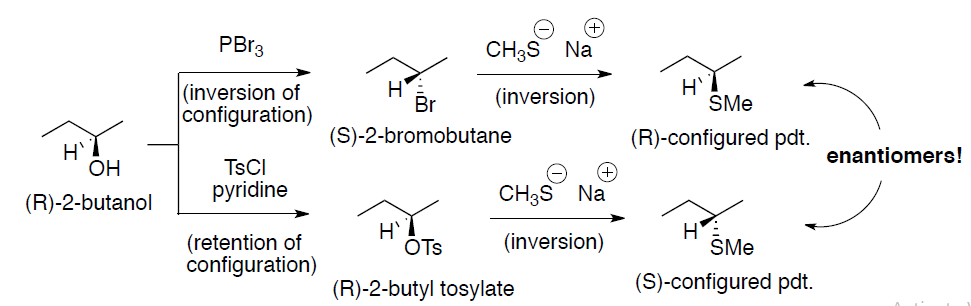
(b) Elimination:
For review papers and other articles,
refer to the tab "References"
Useful Links on Reagent & Reaction:
Mechanism
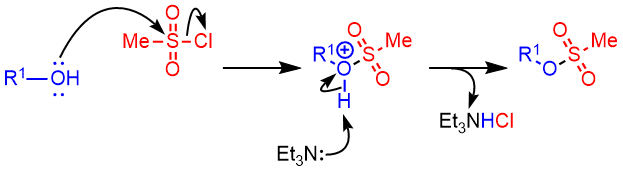
Alcohol to Tosylate – Mechanism
The mechanism of tosylate formation is same as that of mesylate
Additional details
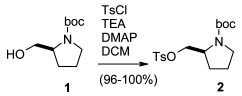
General Procedure:
To a solution of alcohol (1 eq.) in dry DCM (10 Vol) at 0 oC was added pyridine (or) triethylamine (1.5 eq.) followed by p-toluenesulfonyl chloride (1.2 eq.) and stirred at 0 oC for 4 h (If the reaction does not proceed, it shall be brought to room temperature and stirred for 2 h). The reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is diluted with water and layers separated. The aqueous layer is extracted with DCM. The organic layer is then successively washed with water (10 Vol x 2) and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure to get the desired compound.
Note:
- The preferable solvents are DCM. THF and toluene shall also be used.
- If the alcohol is not reactive, in place of py or Et3N, n-BuLi or NaH shall be used to generate the anion.
Typical Procedure:
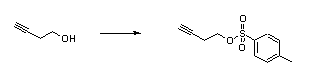
- Tosylation of an alcohol (ChemSpider) — Open access
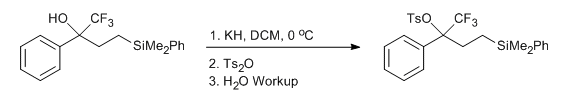
- Tosylation of a Trifluoromethyl Carbinol (ChemSpider) — Open access
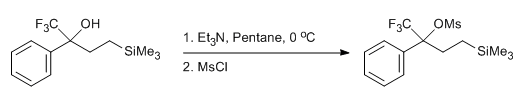
- Mesylation of a Trifluoromethyl Carbinol (ChemSpider) — Open access
- Alcohol to Tosylate-1 (OrgSyn) — Open access
- Alcohol to Tosylate-2 (OrgSyn) — Open access
- Alcohol to Tosylate-3 (OrgSyn) — Open access
- -NH2 to -NHTs-4 (OrgSyn) – N-Tosylates are also synthesized, as similar to O-Tosylates — Open access
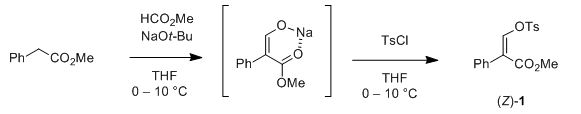
Patent references
Green Chem.
Tosylates have been synthesized on large-scale and they are key intermediates in the manufacturing of pharmaceutical intermediates
Scale-Up Typical Procedure:
- Development of a Scalable Synthetic Process for DG-051B, A First-in-Class Inhibitior of LTA4H (OPRD,2009) – 1.43 Kg batch of alcohol derivative; 1.49 Kg of Tosyl chloride is used
- Kilogram Synthesis of a Selective Serotonin Reuptake Inhibitor (OPRD, 2008) – 3.44 Kg batch; 4.16 Kg of Tosyl chloride is used. n-Tosylation is also done, as similar to O-Tosylation (though it is used for protection-deprotection strategies)
Green Chemistry Aspects: