& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
- Fischer Esterification: It involves the conversion of acid to esters under acidic conditions
- It is a reversible reaction. In order to push the reaction to the forward reaction, a large excess of alcohol is used and the by-product (water) is removed by azeotropic distillation
Advantages
- Inexpensive oxidation method on manufacturing scale
Disadvantages
- It is useful, only if alcohol is inexpensive and commercially available.
- The reaction is reversible and rate of the reaction is slow
Useful Links on Reagent & Reaction:
- Fischer Esterification (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
Fischer Esterification – Mechanism:
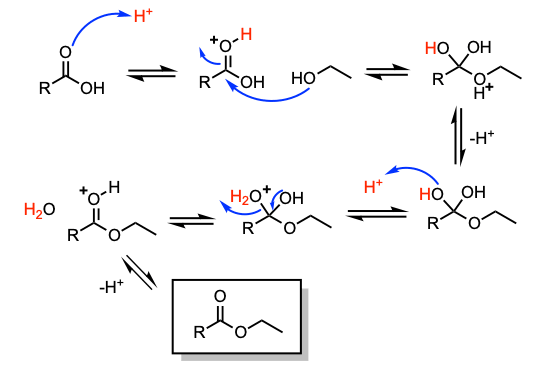
Additional details

General Procedure:
To a solution of carboxylic acid (1 eq.) in alcohol (10 Vol) at 0 oC is added few drops of Conc. H2SO4 (about 5 drops) and stirred at room temperature for 4 h. The reaction is monitored by TLC. If the reaction does not proceed, it shall be refluxed for 4 to 6 h. After the completion of the reaction, the reaction mixture is distilled off and diluted with DCM or EtOAc. The organic layer is then successively washed with water (10 Vol x 2), and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography
Note:
- This reaction is very useful, if alcohol is inexpensive and commercially available. Methyl and Ethyl esters could be prepared easily by this method
- In place of Conc. H2SO4, PTSA could also be used
- This reaction is in equilibrium, though is more favoured. Water (i.e. moisture) tends to hydrolyze the ester back to acid. In order to drive it to the forward reaction, high-temperature reflux, Dean-Stark apparatus and molecular sieves are usually used
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
Patent references
Green Chem.
- Fischer esterification is very useful, if alcohol is inexpensive and commercially available. Methyl and Ethyl esters could be prepared easily by this method on manufacturing scale.
- In place of Conc. H2SO4, PTSA could also be used
Scale-Up Typical Procedure:
