& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
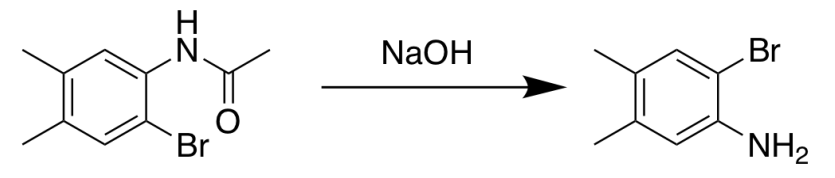
- Amide hydrolysis is carried out by heating either with dilute acid (e.g dil. HCl) or with an alkali (e.g: NaOH solution).
Useful Links on Reagent & Reaction:
Mechanism
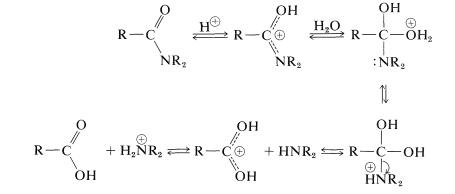
Amide Hydrolysis (acidic condition) – Mechanism
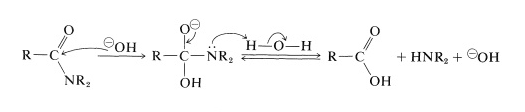
Amide Hydrolysis (basic condition) – Mechanism
Additional details
General Procedure:
To a solution of amide (1 eq.) in MeOH or EtOH (10 Vol) is added 10% aq. NaOH or KOH (2 Vol) and stirred for 16 h The reaction is monitored by TLC. (If the reaction does not proceed, it shall be heated to 60 oC) . The reaction mixture is concentrated under reduced pressure and the residue diluted with water (10 Vol) and extracted with DCM (10 Vol x 2). The aqueous layer is cooled, acidified to pH 3 using 1 N HCl and extracted with DCM (2 x 5 Vol). The combined organic layer is then successively washed with water (10 Vol) and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure to get the desired compound. The crude product is purified by column chromatography.
Note:
- The solvent is EtOH or MeOH, along with water
- Most common bases are KOH and NaOH.
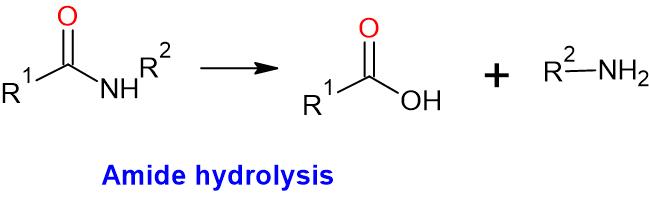
- The main products of amide hydrolysis are carboxylic acid and amine.
Typical Procedure:
WO2007128568, page No. 52 (to change)
Green Chem
Amide Hydrolysis could easily be performed manufacturing scale.
Scale-Up Typical Procedure: