Principles
Solvents
Reagents
Resources
Principles
Green Chemistry is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture, and application of chemical products
12 Principles of Green Chemistry:
1. Prevent Waste
Design chemical syntheses to prevent waste. Leave no waste to treat or clean up.
2. Maximize atom economy
Design syntheses so that the final product contains the maximum proportion of the starting materials. Waste few or no atoms.
3. Design less hazardous chemical syntheses
Design syntheses to use and generate substances with little or no toxicity to either humans or the environment.
4. Design safer chemicals and products
Design chemical products that are fully effective yet have little or no toxicity.
5. Use safer solvents and reaction conditions
Avoid using solvents, separation agents, or other auxiliary chemicals. If you must use these chemicals, use safer ones.
6. Increase energy efficiency
Run chemical reactions at room temperature and pressure whenever possible.
7. Use renewable feedstocks
Use starting materials (also known as feedstocks) that are renewable rather than depletable. The source of renewable feedstocks is often agricultural products or the wastes of other processes; the source of depletable feedstocks is often fossil fuels (petroleum, natural gas, or coal) or mining operations.
8. Avoid chemical derivatives
Avoid using blocking or protecting groups, or any temporary modifications if possible. Derivatives use additional reagents and generate waste.
9. Use catalysts, not stoichiometric reagents
Minimize waste by using catalytic reactions. Catalysts are effective in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and carry out a reaction only once.
10. Design chemicals and products to degrade after use
Design chemical products to break down to innocuous substances after use so that they do not accumulate in the environment.
11. Analyze in real-time to prevent pollution
Include in-process, real-time monitoring, and control during syntheses to minimize or eliminate the formation of byproducts.
12. Minimize the potential for accidents
Design chemicals and their physical forms (solid, liquid, or gas) to minimize the potential for chemical accidents, including explosions, fires, and releases to the environment.
Solvents
In chemical manufacturing process, the selection of solvents plays a crucial role, as solvents are used in very high volumes. The extensive use of these solvents poses the significant health risks and environmental impacts, as most of them are flammable and toxic.
Therefore, it is important to select the solvents with low toxicity, minimal safety concerns, and little impact on the environment to be a greener manufacturing process.
Solvent Selection Guides:
- CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016,18, 288-296 (Original article)– Open access
The Innovative Medicines Initiative (IMI)-CHEM21 public-private partnership is a European consortium which promotes sustainable biological and chemical methodologies. It comprises six pharmaceutical companies from the European Federation of Pharmaceutical Industries and Associations (EFPIA) ten universities and five small to medium enterprises.
CHEM 21 Solvent Selection Guide (classical solvents):
These rankings are defined as below:
– Recommended (or preferred): solvents to be tested first in a screening exercise, if of course there is no chemical incompatibility in the process conditions.
– Problematic: these solvents can be used in the lab or in the Kilolab, but their implementation in the pilot plant or at the production scale will require specific measures, or significant energy consumption.
– Hazardous: the constraints on scale-up are very strong. The substitution of these solvents during process development is a priority.
– Highly hazardous: solvents to be avoided, even in the laboratory.
| Family | Solvent | Ranking |
|---|---|---|
| Water | Water | Recommended |
| Alcohols | MeOH | Recommended |
| EtOH | Recommended | |
| i-PrOH | Recommended | |
| n-BuOH | Recommended | |
| t-BuOH | Recommended | |
| Benzyl alcohol | Problematic | |
| Ethylene glycol | Recommended | |
| Ketones | Acetone | Recommended |
| MEK | Recommended | |
| MIBK | Recommended | |
| Cyclohexanone | Problematic | |
| Esters | Methyl acetate | Problematic |
| Ethyl acetate | Recommended | |
| i-PrOAc | Recommended | |
| n-BuOAc | Recommended | |
| Ethers | Diethyl ether | Highly Hazardous |
| Diisopropyl ether | Hazardous | |
| MTBE | Hazardous | |
| THF | Problematic | |
| Me-THF | Problematic | |
| 1,4-Dioxane | Hazardous | |
| Anisole | Recommended | |
| DME | Hazardous | |
| Hydrocarbons | Pentane | Hazardous |
| Hexane | Hazardous | |
| Heptane | Problematic | |
| Cyclohexane | Problematic | |
| Me-cyclohexane | Problematic | |
| Benzene | Highly Hazardous | |
| Toluene | Problematic | |
| Xylenes | Problematic | |
| Halogenated | DCM | Hazardous |
| Chloroform | Highly Hazardous | |
| CCl4 | Highly Hazardous | |
| DCE | Highly Hazardous | |
| Chlorobenzene | Problematic | |
| Aprotic polar | Acetonitrile | Problematic |
| DMF | Hazardous | |
| DMAc | Hazardous | |
| NMP | Hazardous | |
| DMPU | Problematic | |
| DMSOc | Problematic | |
| Sulfolanec | Hazardous | |
| HMPA | Highly Hazardous | |
| Nitromethane | Highly Hazardous | |
| Miscellaneous | Methoxy-ethanol | Hazardous |
| Carbon disulfide | Highly Hazardous | |
| Acids | Formic acid | Problematic |
| Acetic acid | Problematic | |
| Ac2O | Problematic | |
| Amines | Pyridine | Hazardous |
| TEA | Hazardous |
GSK’s Solevnt Selection Guide:
Reagents
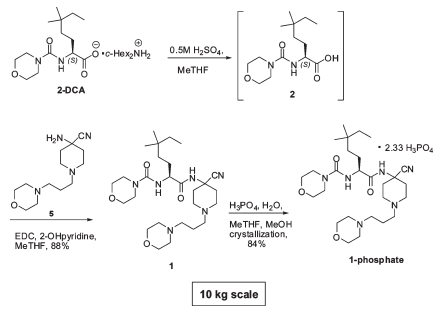
Replacement of expensive and potentially explosive HOBt
- 2-Hydroxypyridine is shown to replace expensive and explosive HOBt
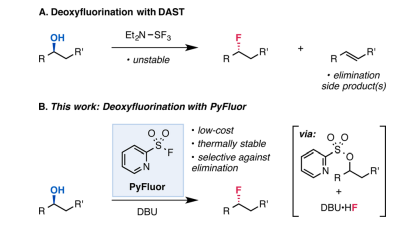
Replacement for explosive DAST
- PyFluor (Pyridine-2-sulfonyl fluoride) is a stable deoxyfluorinating agent, replacing conventional DAST.
- DAST is corrosive, flammable and can be explosive
- PyFluor serves as a reliable reagent for the fluorination of alcohols, offering outstanding stability and a simple reaction procedure
- It enhances selectivity against elimination reactions, facilitating efficient and straightforward purification
Resources
ACS Green Chemistry Institute – Reagents Guide:
- One of the best green chemistry web pages, providing comprehensive information on lot of reagents – A wealth of knowledge is available here
GSK’s Reagent Guide: