& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
- DCC is Dicyclohexylcarbodiimide
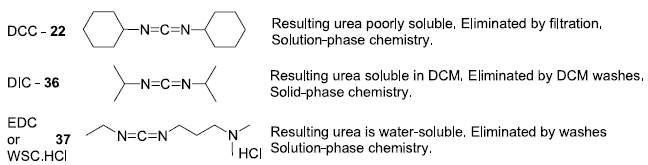
- While using DCC as a coupling agent,, DUC (Dicyclohexylurea) is formed as a by-product, which is difficult to remove using column chromatography
- DUC, being insoluble in water, can not be removed during aqueous work-up. However, an alternate reagent namely, EDCI and its urea by-product are soluble in water and hence they can be eliminated in the work-up using aqueous washings.
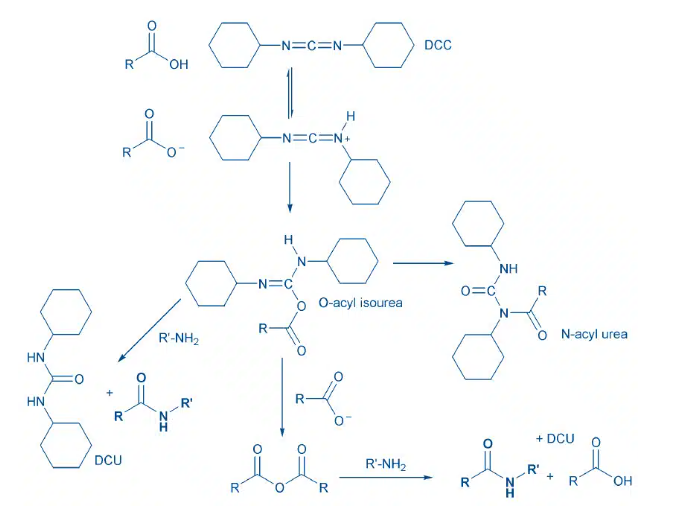
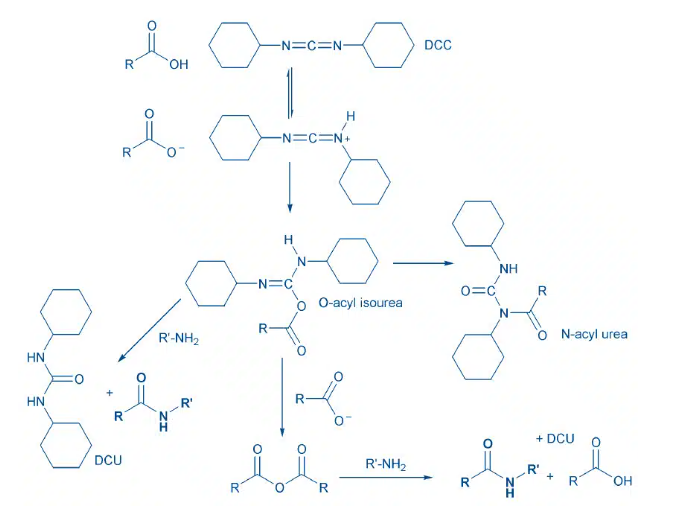
- Carbodiimide reacts with carboxylic acid to form O-acylisourea, an activated intermediate. The amine reacts with O-acylurea to result in amide
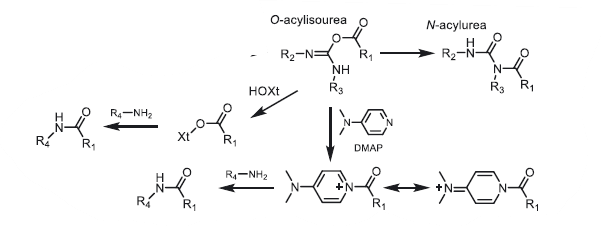
- Side-product formation: The O-acylisourea can also rearrange by intramolecular acyl transfer to give the N-acylurea, which is an irreversible pathway that does not lead to desired amide. Additives (e.g: HOBt, HOAt) are usually used along with carbodiimides to avoid side-product formation
Commonly used carbodiimides on scale:

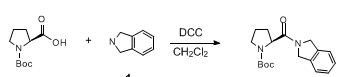
Acid-Amine Coupling by DCC
Additives or Activators:
- Additives or activators such as HOBt, HOAt, HOSu or Oxyma pure® are strongly recommended in all cases of amide bond formations with carbodiimides.
- These additives are helpful to enhance the reactivity and also to reduce formation of epimers as well as N-acylureas.
Mechanism of activation by HOBt when used as an additive with DCC
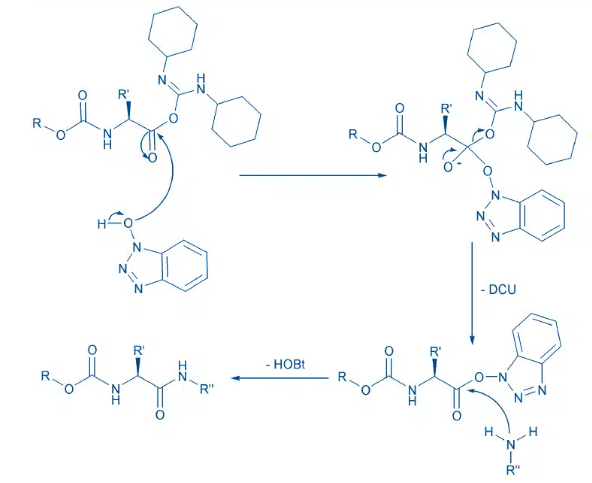
- As an alternative to the use of N-hydroxylamines (HOBt or HOAt), catalytic amount of a tertiary amine (DMAP) could also be used to favor the progress of the reaction

- HOBt and HOAt have explosive character, especially in water-free form
- HOBt and HOSu are commonly used additives. HOAt is very expensive
- HOSu is completely shelf-stable. A drawback of HOSu is due to its hydroxamic acid structure susceptible to the Lossen-rearrangement. This side reaction can be observed under condensation conditions and leads to the introduction of an additional β-alanine.
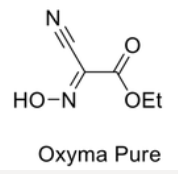
Oxyma Pure® (Ethyl 2-cyano-2-(hydroximino)acetate)
- A recently developed additive and is trademark of Luxembourg Bio Technologies Ltd, Rechovot, Israel
- Oxyma Pure®is a non-explosive alternative to HOBt or HOAt, and allows high coupling rates at low racemization when applied in combination with carbodiimides.
Other Uses of DCC:
- As a dehydrating agent
- in the preparation of esters, especially between tertiary alcohols and carboxylic acid
- Used in oxidation of alcohols to aldehydes and ketones – Pfitzner-Moffatt Oxidation (DMSO, DCC)
Mechanism
Acid-Amine coupling by DCC – Mechanism
- Carbodiimide react carboxylic acid to form O-acylisourea, an activated intermediate. The amine reacts with O-acylurea to result in amide
- Alternatively, excess carboxylic acid can react with the O-acylisourea to form the symmetrical anhydride, which is also a good acylating agent. Acylation of an amine with the symmetrical anhydride also produces amide.
- Side-product formation: The O-acylisourea can also rearrange by intramolecular acyl transfer to give the N-acylurea, which is an irreversible pathway that does not lead to desired amide
Acid-Amine coupling by Carbodiimides – General Mechanism

Additional details
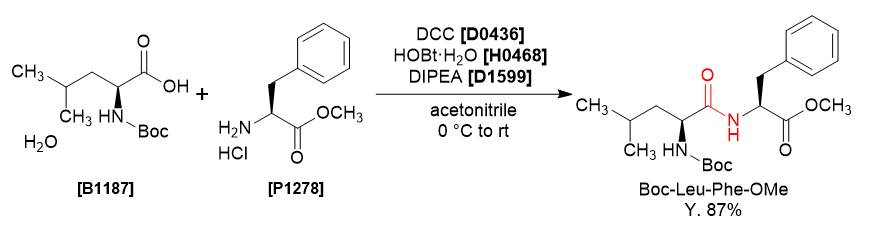
Acid-Amine Coupling by DCC;

General Procedure-1:
The carboxylic acid (1 equiv.), amine (1 eq.), N,N-Dicyclohexylcarbodiimide (DCC, 1 eq.), and DMAP (0.3 eq.) are dissolved in dichloromethane at room temperature and the mixture is stirred at RT for 12 hours. The reaction is monitored by TLC. The resulting mixture is filtered and the filtrate is added with water. The layers are separated and the aqueous layer is extracted with DCM (10 Vol). The organic layers are combined and washed with water (10 Vol) and brine solution (5 Vol). The resultant organic layer is dried over Na2SO4 and concentrated. The crude product is purified by column chromatography
General Procedure-2:
A stirred, cooled solution of carboxylic acid (1 eq.) in ethyl acetate (10 Vol) at 0 oC is treated dropwise with N-Hydroxy succinimide (NHS,1 eq.) and N,N-Dicyclohexylcarbodiimide (DCC, 1 eq.) and stirred at 0 oC for one hour. A solution of amine (1.1 eq.) in ethyl acetate (10 Vol) iss added at 0 oC and stirred at room temperature for 4-6 h. The reaction is monitored by TLC.
The reaction mixture is filtered (to remove the byproduct urea derivative) and the filtrate is washed with water. The organic layer is washed with brine solution, dried over anhydrous sodium sulphate and concentrated. The crude material is purified by flash column chromatography using 8:2 ethyl acetate-hexane system to afford the desired compound
Note:
- The most preferable solvents are DCM, THF and Ethyl acetate. The reaction is usually done at RT
- The by-product from DCC namely Dicyclohexylurea (insoluble in most of the solvents) is usually filtered.
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Acid-Amine coupling by DCC (OrgSyn) — Open access
- Condensation Using DCC and HOBt (TCI) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
Patent references
Green Chem
DCC has widely been used for manufacturing of pharmaceuticals
Advantages:
- DCC is a low melting white solid (m.pt=34 oC). The low melting point allows it to be melted for easy handling in liquid form
- It is inexpensive
- It is soluble in organic solvents (DCM, THF, DMF, CH3CN). However, it is insoluble in water
Disadvantages:
- The insoluble by-product (DUC, Dicyclohexylurea) poses challenges during the isolation and purification
- Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals (OPRD, 2016) – Excellent review article, providing comprehensive information on large-scale reactions of coupling reagents