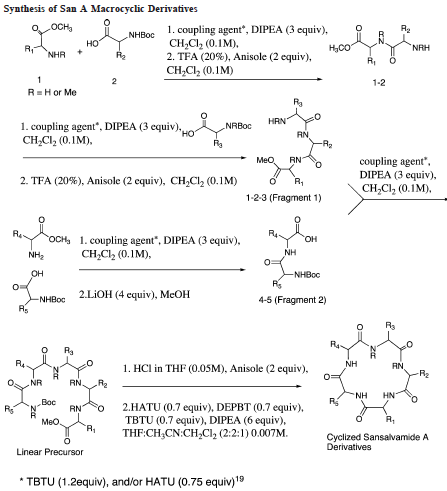
& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info

- TBTU is very similar to HBTU with respect to structure except for the nature of salt (BF4 salt is TBTU and PF6 salt is HBTU)
- Uronium salts are useful for the coupling of sterically hindered amino acids in peptide synthesis
- When the carboxylic acid contains epimerizable α-stereocenter, 1 eq. of HOAt or HOBt shall be used to suppress epimerization.
Disadvantages of Uronium salts:
- The by-product from these uronium salts, namely N,N,N′,N′-tetramethylurea are cytotoxic
- The presence of high energy moiety (Benzotriazole) makes it less attractive for large-scale reactions – Mild reaction conditions should only be maintained
- As the molecular weight is high, quantities required would be higher, thereby higher cost per mole
Comparison of HATU, HBTU and TBTU from Scale-up perspective: Refer to the tab “Scale-up & Green Chem”
Commonly used Uronium salts:
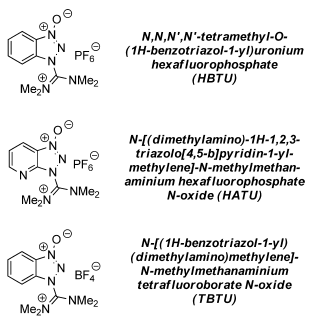
- The counterion in the above salts has no influence on the reactivity
Side-product formation while using HBTU or HATU or TBTU:
- Uronium species are also known to be guanidylation agents as well

- The side-product formation shall can be diminished by adding HOBt to the reaction (similar to HOBt in DCC)


- HOBt and HOAt have explosive character, especially in water-free form
- HOBt and HOSu are commonly used additives. HOAt is very expensive
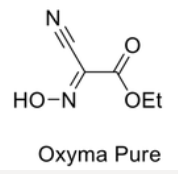
Oxyma Pure® (Ethyl 2-cyano-2-(hydroximino)acetate)
- A recently developed additive and is trademark of Luxembourg Bio Technologies Ltd, Rechovot, Israel
- Oxyma Pure®is a non-explosive alternative to HOBt or HOAt, and allows high coupling rates at low racemization when applied in combination with carbodiimides.
Mechanism
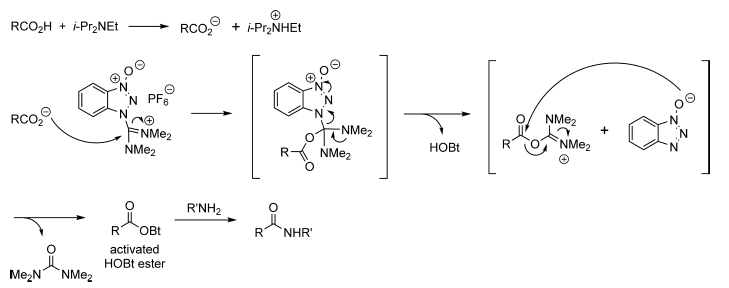
Acid-Amine coupling by TBTU – Mechanism
- The mechanism of TBTU is same as that of HBTU (hence mechanism of HBTU is mentioned below for reference)
- The first step is the formation of carboxylate anion with bases such as Et3N or DIPEA
- The next step is the formation of activated HOBt ester by the reaction between carboxylate anion and TBTU. The by-product of TBTU viz., N,N,N′,N′-tetramethylurea is liberated here.
- HOBt ester gets attached by amine to give amide
Additional details
Acid-Amine Coupling by TBTU;

General Procedure:
To a solution of acid (1 eq.) in CH2Cl2 (10 Vol) at 0 °C was added TBTU (1 eq.) and DIPEA (2 eq.). Amine (1 eq.) was added slowly to this mixture. The RM was then brought to RT and stirred overnight. The reaction is monitored by TLC. The organic layer is diluted with CH2Cl2 (10 Vol) and then successively washed with water (2 x 5 Vol) and brine solution (5 Vol). The resultant organic layer is dried using sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography.
Note:
- TBTU is 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate
- The most preferable solvent is DCM. Other solvents are DMF
- In place of DIPEA, 2,6-Lutidine could also be used
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2007127635, page No. 173
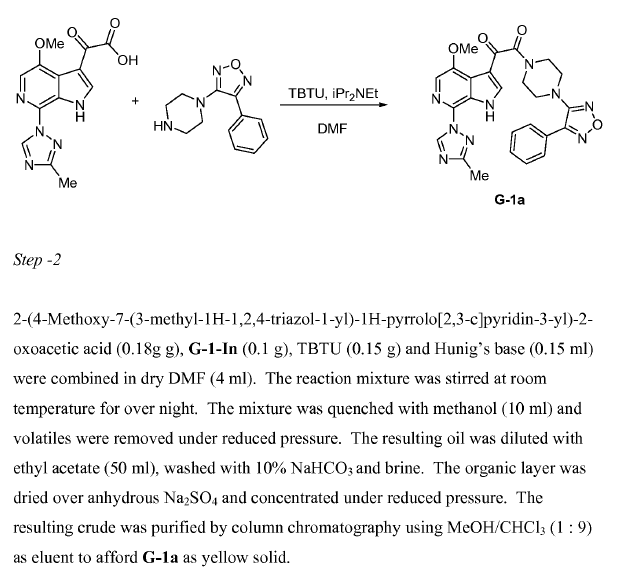
WO2010038081, page No. 678
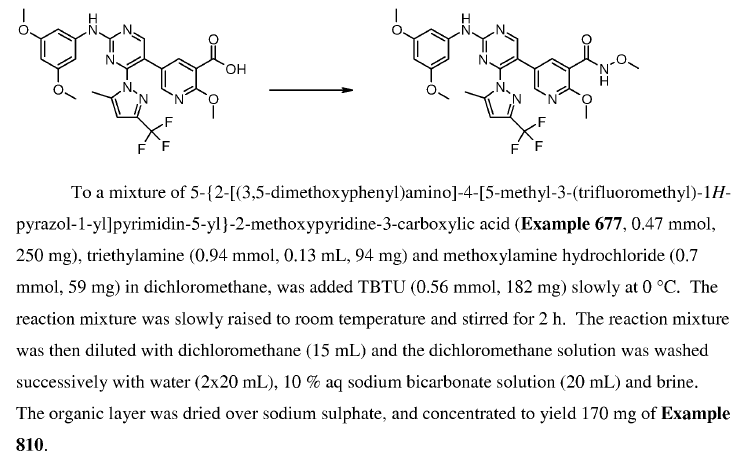
WO2010038081, page No. 600
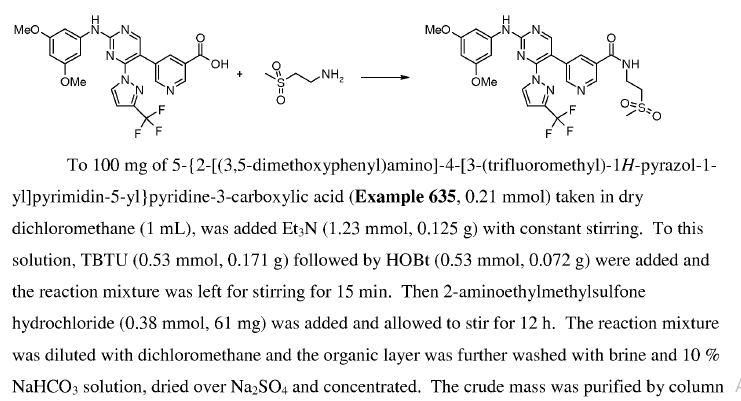

Green Chem
- TBTU and HATU are used for process development, though HBTU is not used widely in process chemistry,
- Both HATU and TBTU provide faster couplings, reduce epimerization and result in high yields (Ref: OPRD, 2016, 140-177)
Scale-Up Typical Procedure:
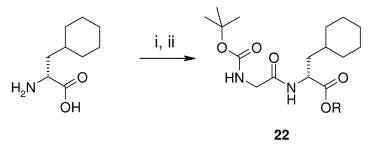
- Selection and Development of a Route for Cholesterol Absorption Inhibitor AZD4121 (OPRD, 2012) – 168 g of TBTU is used; 84.5 g batch (N-Boc glycine)
(i) thionyl chloride, methanol, 83%; (ii) N-Boc-Gly-OH, TBTU, NMM, DCM, 23 °C, 85%
- Large-Scale Applications of Amide Coupling Reagents for the Synthesis of Pharmaceuticals (OPRD, 2016) – Excellent review article, providing comprehensive information on large-scale reactions of coupling reagents