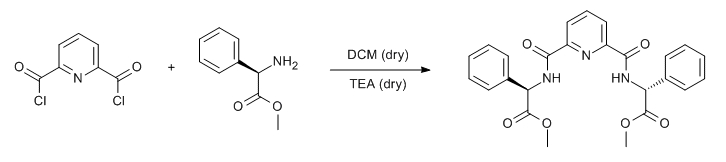
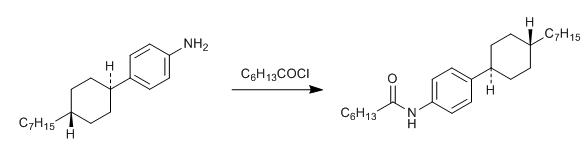
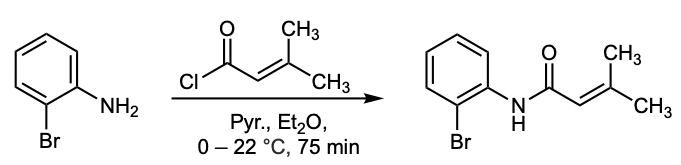
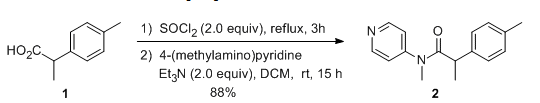
& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
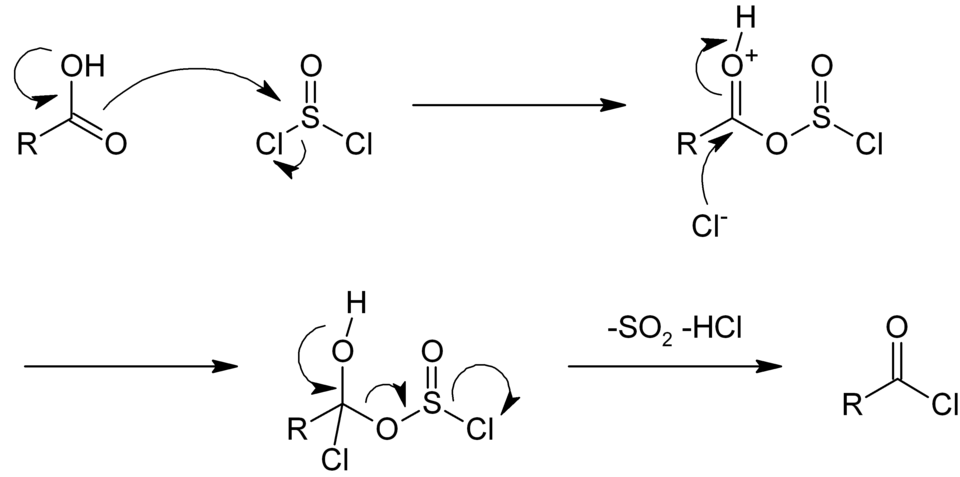
- Thionyl chloride (SOCl2) is commonly used to convert (a) acid to acid chloride and (b) alcohol to Chloride
- Other reagents that convert alcohols to chloride are oxalyl chloride, (COCl)2, PCl3 and PCl5
Advantages
- Inexpensive method on manufacturing scale
- The by-products liberated during the reaction are HCl and SO2, As both being gases, the reaction is usually clean
Disadvantages
- Similar to other sulphur compounds, handling SOCl2 is bit difficult, due to its peculiar unpleasant pungent smell.
- Handling of SOCl2 needs thorough safety studies before planning manufacturing scale
Useful Links on Reagent & Reaction:
- Deoxychlorination (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry information on various chlorinating reagents.
For review papers and other articles,
refer to the tab "References"
Mechanism
Mechanism
Additional details
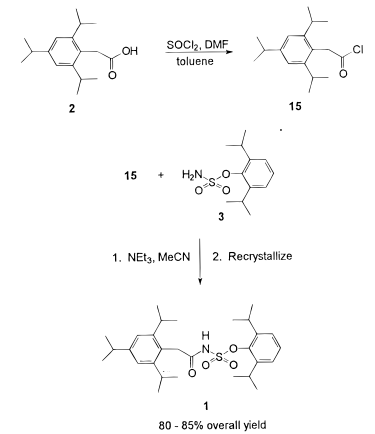
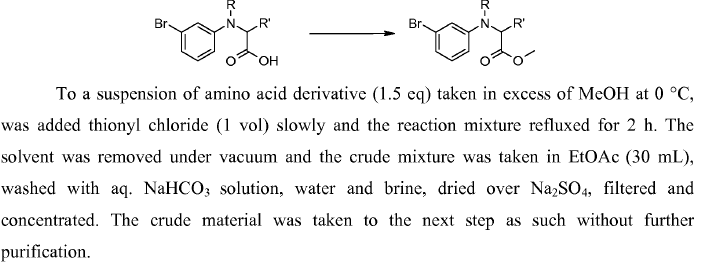
General Procedure-1:
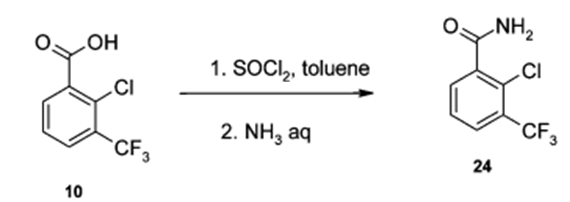
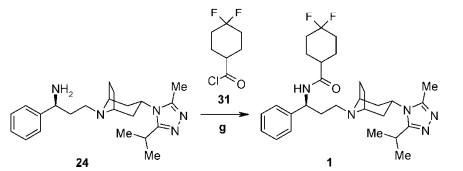
(a) Acid chloride preparation:
To a solution of carboxylic acid (1 eq.) in DCM or THF or toluene (10 Vol) at 0 oC is added SOCl2 (2 – 3 eq.) and stirred at room temperature overnight (If required, catalytic amount of DMF shall be added). The reaction is monitored by TLC (If the reaction does not proceed, it shall be heated) The acid chloride formation is confirmed by adding reaction mixture to methanol and TLC is verified (whether it forms ester or not). After the completion of the reaction, excess thionyl chloride is distilled off to get the residue. (This residue is acid chloride and should be used as soon as it is prepared, as acid chlorides are usually very reactive and gets hydrolyzed on contact with moisture)n
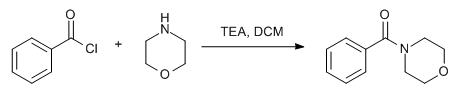
(b) Acid chloride to amide:
resultant residue (i.e. acid chloride) is dissolved in DCM or THF. To this solution is added amine (1.2 eq.), followed by base (py or DMPA or DIPEA) and stirred at room temperature for 4h. The reaction is monitored by TLC. The reaction mixture is then successively washed with water (10 Vol x 2), aq. HCl solution and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography
Note:
- The preferable solvents are DCM, THF or toluene
- During acid chloride, the by-products liberated are HCl and SO2, As both being gases, the acid chloride reaction is usually clean.
- If the acid chlorides are available commercially, it shall be used as such, following procedure (b).
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Secondary aromatic amides from anilines (ChemSpider) — Open access
- Acid to Amide via acid chloride-1 (OrgSyn) — Open access
- Acid to Amide via acid chloride-2 (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2010038081, page No. 203

WO2010038081, page No. 152
Green Chem.
- Synthesis of amides from carboxylic acids via 2 steps (acid chloride followed by amine) is one of common methods adopted for manufacturing
- Thionyl chloride could be carried out on large-scale. However, The by-products liberated during the reaction are HCl and SO2, Appropriate safety controls are to be ensured while performing manufacturing.
- Inexpensive method on manufacturing scale
Scale-Up Typical Procedure:
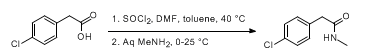
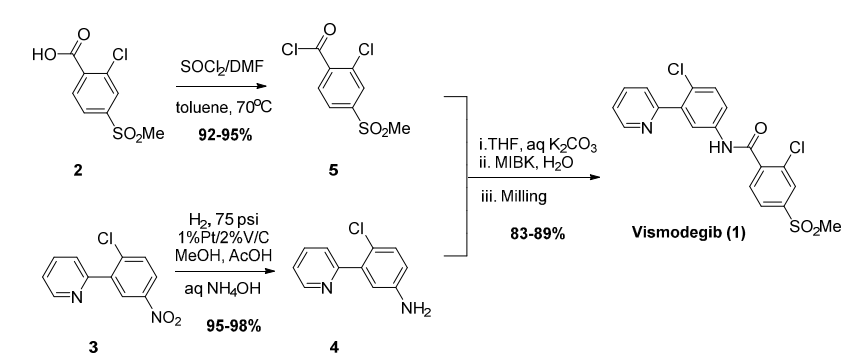
- Manufacturing Development and Genotoxic Impurity Control Strategy of the Hedgehog Pathway Inhibitor Vismodegib (OPRD, 2016) – (a) 23 Kg batch (acid); 13.9 Kg SOCl2 (b) 18.9 Kg acid chloride; 13.9 Kg amine
- Concise Preparation of a Stable Cyclic Sulfamidate Intermediate in the Synthesis of a Enantiopure Chiral Active Diamine Derivative (OPRD, 2015) – 898 g batch; 584 mL of SOCl2 is used
- Development of a Bulk Enabling Route to Maraviroc (UK-427,857), a CCR-5 Receptor Antagonist (OPRD, 2008) – 1.54 Kg acid chloride is used
- Chemical Development of a Pilot Scale Process for the ACAT Inhibitor 2,6-Diisopropylphenyl [(2,4,6-Triisopropylphenyl)acetyl]sulfamate (OPRD, 1997) – 33.9 Kg batch; 18.5 Kg of SOCl2 is used
Green Chemistry Aspects:
- Deoxychlorination (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry information on various chlorinating reagents.