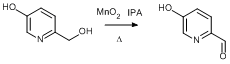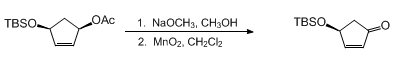& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
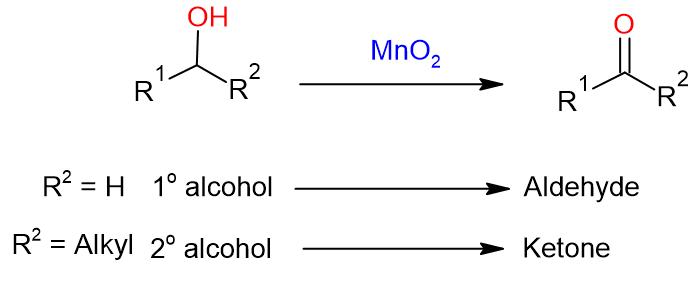
- MnO2 (Manganese dioxide) (DMSO, Oxalyl chloride) is generally useful to oxidize activated alcohols (such as allylic and benzylic alcohols) to their corresponding aldehydes.
- It is not a suitable reagent for unactivated alcohols
- The reaction is heterogeneous and requires large excess of MnO2
Advantages
- Inexpensive oxidation method on manufacturing scale
- Compared to other oxidising agents, it is a safer alternative
Disadvantages
- A large excess of MnO2 (about 10 X) is usually required
- Freshly prepared MnO2 is usually preferred, as its quality would go down over a period of time.
Useful Links on Reagent & Reaction:
- Manganese Dioxide, MnO2 (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
For review papers and other articles,
refer to the tab "References"
Mechanism
MnO2 mechanism is not very clear. It is believed to undergo via a radical mechanism on the surface of MnO2 particles.
Additional details
General Procedure:
To a solution of Substrate (alcohol, 1 eq.) in dry CH2Cl2 (20 ml) is added MnO2 (20 eq.) and stirred at RT overnight. The reaction is monitored by TLC. The RM was filtered through celite pad and the filtrate was concentrated to give rise to the product.
Note:
- The reaction is heterogeneous and requires large excess of MnO2
- Activated alcohols such as Allylic and Benzylic alcohols are easily oxidized to corresponding aldehydes. However, MnO2 is not suitable for unactivated alcohols.
- Freshly prepared MnO2 is usually preferred, as its quality would go down over a period of time.
Typical Procedure:
- Oxidation of 6-(Hydroxymethyl)pyridin-3-ol (ChemSpider) — Open access
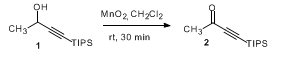
- MnO2 Oxidation-1 (OrgSyn) — Open access
- MnO2 Oxidation-2 (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2010045258, page no. 221
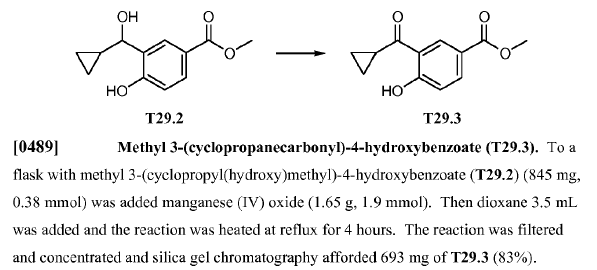
WO2010038081, page no. 819

Green Chem.
MnO2 oxidation could be carried out on large-scale. However, the reaction requires large excess of MnO2.
- MnO2 Oxidation is one of the inexpensive methods to manufacture aldehydes or ketones from Alcohols, though it oxidises only activated alcohols.
- Also, refer to Swern Oxidation (DMSO, Oxalyl chloride) for Scale-Up
Scale-Up Typical Procedure:
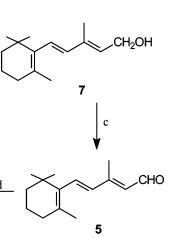
- An Efficient Commercial Process for the Preparation of Isotretinoinxidation (OPRD, 2005) – 1 Kg batch; 3 Kg of MnO2 has been used
Green Chemistry Aspects:
- Permanganate: A Green and Versatile Industrial Oxidant. Org. Proc. Res. Dev. 2001, 5, 6, 599–603 – Waste MnO2 can be recycled to permanganate
MnO2 Oxidation – References: