& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
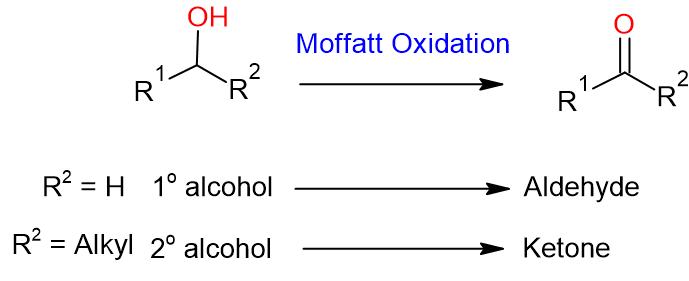
- Moffatt Oxidation (DMSO, DCC, Bronsted acid) is a mild oxidizing system that converts primary alcohol to aldehyde and secondary alcohol to ketone
- The effective Bronsted acid (i.e proton source) is H3PO4 or py.TFA. Usually, HCl, TFA or H2SO4 are not good proton sources for this oxidation
- It is one of the series of oxidations involving DMSO (refer to DMSO-activated oxidations)
Advantages
- Inexpensive oxidation method on manufacturing scale
- Unlike Swern oxidation that requires cryogenic condition (-78 oC), Moffatt oxidation is performed at room temperature
- Also, it is less toxic, compared to chromium-based alternatives (PCC and PDC)
Disadvantages
- The liberation of gas viz., malodrous dimethylsulphide (Me2S) is to be handled appropriately
- Large amounts of DCC is required (usually 3 eq.) that makes the process difficult
- The by-product from DCC namely Dicyclohexylurea could be difficult to remove from the product, unless it is precipitated during the work-up.
- Even if Dicyclohexylurea precipitates from the solution, it is partially soluble enough in solvents, such that it is not fully removed during the filtration
Useful Links on Reagent & Reaction:
- DMSO –DCC (Pfitzner-Moffatt Oxidation) (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
- Pfitzner-Moffat oxidation (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
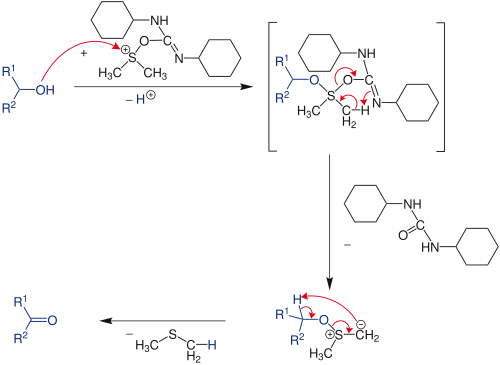
General Oxidation Mechanism
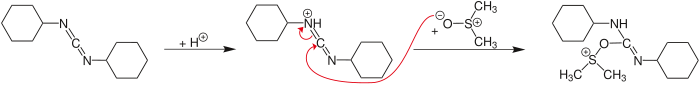
DMSO, as such, is not a good oxidising agent. However, it needs to be activated by an activator [such as (COCl)2 in Swern Oxidation, DCC in Moffatt oxidation] so that DMSO shall act effective oxidising agent (refer to DMSO-activated Oxidations)
Activation of DMSO by DCC
Moffatt Oxidation
Additional details
General Procedure:
To a solution of substrate (alcohol; 1 eq.), DCC (3 eq.) in dry DMSO (5 Vol) is added crystalline orthophosphoric acid (1.5 eq.). The mixture is stirred at RT for 6 h. The reaction is monitored by TLC.
The reaction mixture is then diluted with CH2Cl2 (10 Vol) or EtOAc (10 Vol) and the resultant by-product (Dicyclohexylurea) is filtered. The filtrate is washed with water and layers separated. The aqueous layer is extracted with DCM (10 Vol) or EtOAc (10 Vol). The organic layers are combined and washed with water (10 Vol) and brine solution (5 Vol). The resultant organic layer is dried over Na2SO4 and concentrated. The crude product shall be used for next reaction or if required, is purified by column chromatography.
Note:
- The reaction is usually done at RT
- The unreacted excess DCC shall be removed by adding oxalic acid (i.e. Oxalic acid converts DCC to Dicyclohexylurea)
- Dicyclohexylurea (DCU) is poorly soluble in water but more soluble in organic solvents like ethanol, acetone, dichloromethane, and ethyl acetate
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
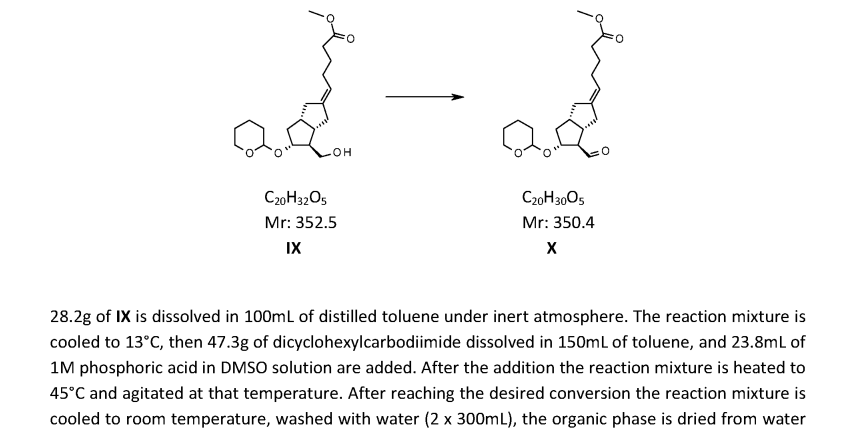
WO2019202345, page no. 36

Green Chem.
Moffatt oxidation is not a convenient reaction on large-scale. However, the reaction involves the liberation of 1 eq. gas, Me2S (dimethylsulphide) and appropriate safety controls are to be ensured while performing manufacturing. This reaction results in a by-product namely Dicyclohexylurea that often poses problems during isolation.
- The liberation of gas viz., malodrous dimethylsulphide (Me2S) is to be handled appropriately
- Unlike Swern oxidation that requires cryogenic condition (-78 oC), Moffatt oxidation is performed at room temperature
Alternative to Pfitzner-Moffatt Oxidation (DMSO, DCC) to avoid insoluble by-product for Scale-Up:
- Parikh-Doering Oxidation (DMSO, py.SO3)
Green Chemistry Aspects:
Process Safety Aspects:
Swern oxidation could be carried out on large-scale. However, the reaction involves the liberation of 1 eq. each of the gases such as Me2S (dimethylsulphide), CO (carbon monoxide), CO2. Appropriate safety controls are to be ensured while performing manufacturing. During work-up, HCl gets converted to amine salt (such as NEt3.HCl).
- Swern Oxidation is one of the inexpensive methods to manufacture aldehydes or ketones from Alcohols
- The liberation of gases viz., malodrous dimethylsulphide (Me2S), poisonous carbon monoxide (CO) and CO2 are to be handled appropriately
- It is important to maintain the reaction mixture at -78 oC. If the temperature is not maintained, there is a possibility of formation of mixed thioacetals (see mechanism in General Info section)
Activated DMSO Oxidation – Review: