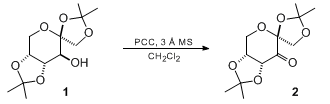
& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info

- PCC (Pyridinium Chlorochromate) is also called Corey’s reagent
- It is a chromium salt, synthesized from pyridinium hydrochlride and CrO3
- PCC is a milder reagent than Chromic acid and hence it will not oxidise aldehydes to carboxylic acids.
- By virtue of not having water in the system, PCC does not oxidise aldehyde to carboxylic acids, unlike chromic acid (H2CrO4) that further oxidises -CHO to -COOH
- PCC oxidation reaction is to be performed under anhydrous condition
Advantages
- NIL
Disadvantages
- Being a chromium salt, PCC is toxic
- PCC is acidic and hence it is not suitable for acid-labile substrates.
- PCC is the least preferred oxidising agent for the conversion of alcohol to aldehyde while Green Chemistry is taken into consideration
Useful Links on Reagent & Reaction:
- PCC (Corey’s reagent) (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
For review papers and other articles,
refer to the tab "References"
Mechanism
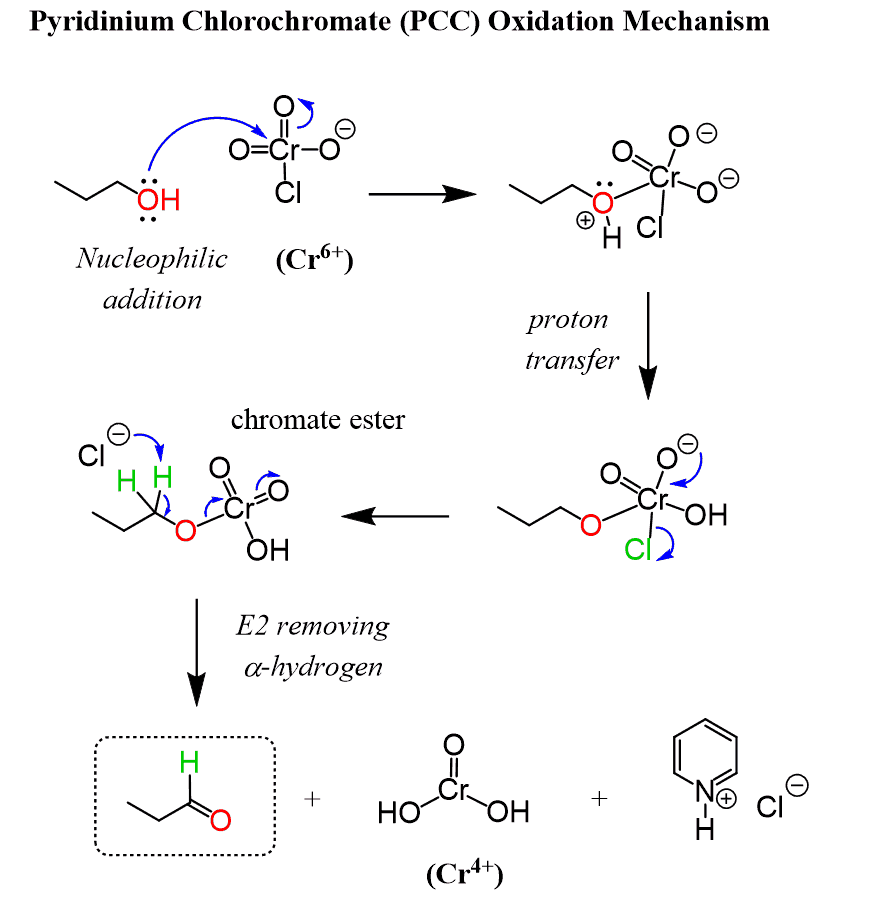
Image Credit to “chemistrysteps.com”
Additional details
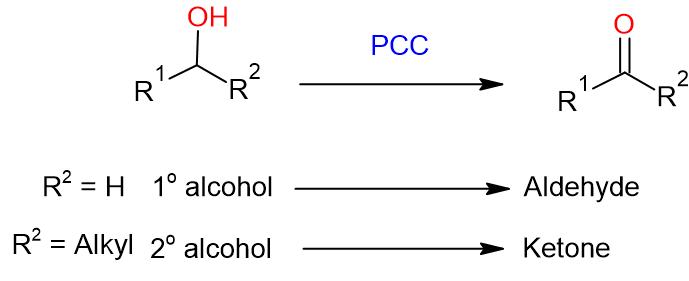
General Procedure:
To a solution of substrate (alcohol; 1 eq.) in CH2Cl2 (5 Vol) is added Pyridinium chlorochromate (1.2 eq.) in CH2Cl2 (5 Vol) and Celite at 0 oC and stirred at RT for 2 to 4 h. The reaction is monitored by TLC.
During the reaction progress, a brown tar-like material slowly gets precipitated out. The reaction mixture containing solids is filtered through Celite bed and the bed washed with CH2Cl2. The organic layers are combined, and washed with water (10 Vol) and brine solution (5 Vol). The resultant organic layer is dried over Na2SO4 and concentrated. The crude product shall be used for next reaction or if required, is purified by column chromatography
Note:
- PCC oxidation reaction is to be performed under anhydrous condition
- The addition of Celite or Molecular sieves helps in PCC reaction to make the brown tar-like material (side products from the reagent) not sticking to the bottom of the flask.
- The most preferable solvent is DCM
- PCC is acidic and hence it is not suitable for acid-labile substrates
- PCC is a toxic reagent
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- PCC Oxidation-1 (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2005023787, page No. 5 (100 g batch)



Green Chem.
PCC is not a suitable oxidizing agent for larger scale reactions owing to following reasons:
- Being a chromium salt, PCC is toxic
- PCC is the least preferred oxidising agent for the conversion of alcohol to aldehyde while Green Chemistry is taken into consideration
Scale-Up Typical Procedure:
- A Facile and Scaleable Synthesis of 3-O-Decladinose-6-methyl-10,11-dehydrate-erythromycin-3-one-2‘-acetate, an Important Intermediate for Ketolide Synthesis (OPRD, 2006) – 196 g batch; 396 g of PCC has been used

Alternative to PCC oxidation for Scale-Up:
- Parikh-Doering Oxidation (DMSO, py.SO3)
Green Chemistry Aspects:
Pyridinium Chlorochromate (PCC) – Review: