& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info

- Williamson synthesis is the simplest and most popular method of preparing ethers, widely used in both laboratory and industrial synthesis
- Both symmetrical and unsymmetrical ethers are easily prepared
- Reactant-1 (Alkyl halide): Methyl and 1o alkyl halide (including allyl and benzyl halides)
- Reactant-2 (Alcohol): Aliphatic and aromatic alcohol
- Bases: K2CO3, KtOBu – Depending on the nature of alcohol to generate anion
- Solvents: THF, DMF, DMSO – Depending on the type of base used
- Product: Substitution product containing C-O bond
- Reaction type: SN2
Nature of Alkyl halide
- Key requirement: Alkyl halide must be CH3 or 1o (unhindered alkyl halide)
- Alkyl halide reactivity is in line with SN2 mechanism [Me > 1o > 2o > 3o]
- Me & 1° alkyl halides: most favourable and yields are higher
- 2° halides: competing with beta-elimination; Lower yields
- 3° halides: The reaction fails and results in beta-elimination only; i.e only alkene formation & no ether is formed


Useful Links on Reagent & Reaction:
- Williamson ether synthesis (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
Williamson Ether synthesis – Mechanism
The mechansim involves SN2

Additional details
Williamson Ether Synthesis:

General Procedure-1 (O-alkylaton of Phenols and activated alcohols):
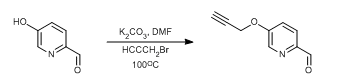
To a suspension of Phenol derivative (1 eq.), Cs2CO3 or K2CO3 (2 eq.) in acetontrile (15 Vol) is added alkyl halide (usually 1o halide) at room temperature and stirred for 6 h (If the reaction does not proceed, it shall be brought to room temperature and stirred for 2 h). The reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is filtered to remove inorganics. The filtrate (i.e. organic layer) is successively washed with water (10 Vol ml x 2) and brine solution (15 ml), dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography.
General Procedure-2 (O-alkylaton of unactivated alcohols):
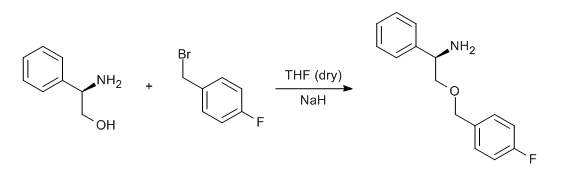
To a suspension of NaH (1.2 e.) in THF (10 Vol) iss added alcohol derivative (1 eq.) at 0 oC and stirred for 1-2 h. A solution of alkyl halide (usually 1o halide) in THF is added at 0 oC and stirred for 4 h (If the reaction does not proceed, it shall be brought to room temperature and stirred for 2 h). The reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is acidified with 6N HCl and extracted with DCM or MTBE two times. The resulatnt organic layer is successively washed with water (10 Vol ml x 2) and brine solution (15 ml), dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography.
Note:
- Alkyl halide should be Methyl and 1o alkyl halide (including allyl and benzyl halides)
- Alcohol shall be Aliphatic and aromatic alcohol
- The nature of solvent is based on the choice of base being used
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Williamson etherification of a bromomethylfluorobenzene and phenylglycinol (ChemSpider) — Open access

- Mono-Alkylation of a Phenol with 1,2-Dibromoethane via Williamson Ether Synthesis (ChemSpider) — Open access

For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2007128568, page No. 59

WO2010045258, page No. 270


Green Chem
Williamson ether synthesis has been carried out on large-scale and there are several reports available in OPRD
Scale-Up Typical Procedure:
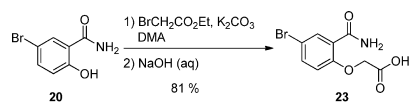
- Process Development and Scale-Up of a Benzoxazepine-Containing Kinase Inhibitor (OPRD, 2015) – 4 Kg batch (Phenol derivative); 3.24 Kg of Ethyl bromoacetate are used
Green Chemistry Aspects: