& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
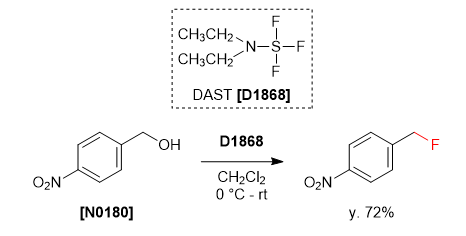
DAST, (Diethylamino)sulfur trifluoride:

- DAST, (Diethylamino)sulfur trifluoride, is a nucleophilic fluorinating agent.
- The reaction is usually performed at 0 oC to -78 oC. In general, DAST reactions should not be heated. Upon heating, DAST converts to the highly explosive (NEt2)2SF2 with expulsion of sulfur tetrafluoride

Advantages:
- Nil
Disadvantages:
- DAST is heat sensitive and moisture sensitive.
- Always look for safer alternatives to avoid DAST. There are several relatively safer reagents available to convert alcohol to fluoride
DAST – Functional Group Conversions

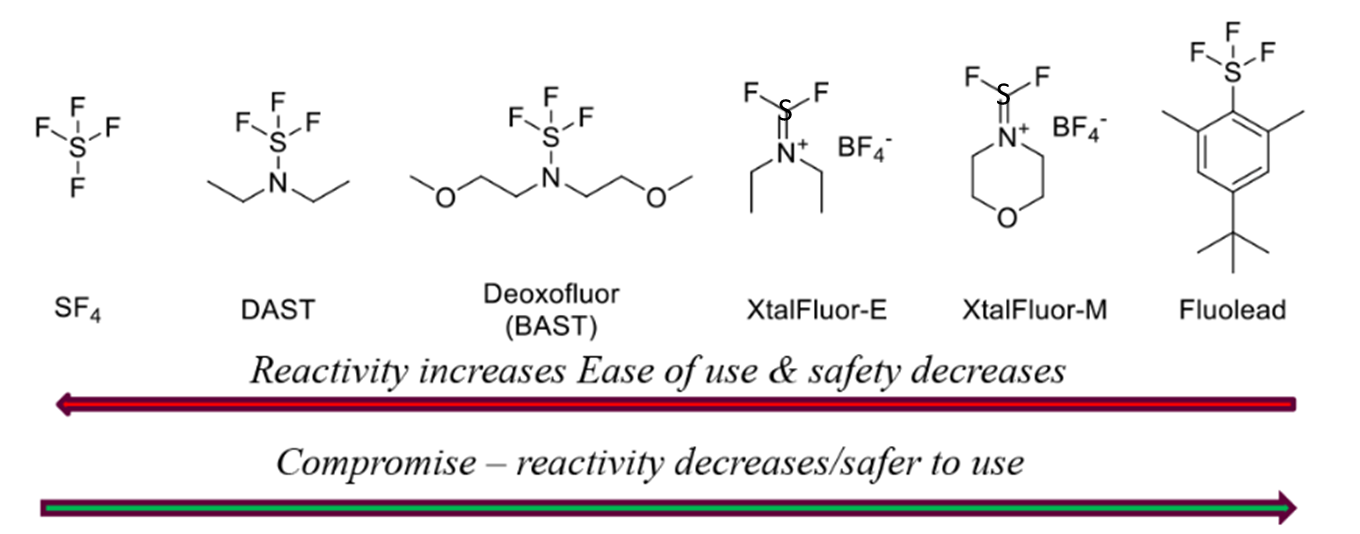
Sulfur-based Deoxyfluorinating reagents
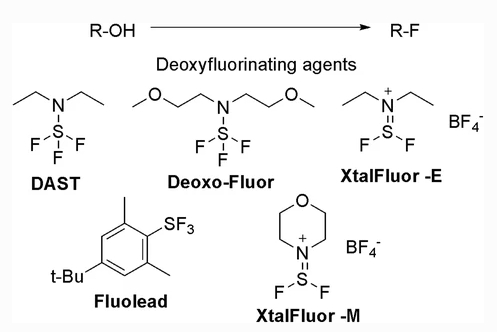
- Stable Fluorinating Reagent with Ease of Handling (FLUOLEAD®) – A safer substitute for DAST
Useful Links on Reagent & Reaction:
- Sulfur-Based Fluorinating Agents (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
For review papers and other articles,
refer to the tab "References"
Mechanism
DAST – Mechanism

Additional details

General Procedure:
To a solution of alcohol or carbonyl compound (1 eq.) in dichloromethane (20 Vol) is added DAST (1.2 eq.) dropwise at –78 oC under nitrogen atmosphere and stirred at room temperature for 2 h. The reaction is monitored by TLC. The reaction mxture is quenched with saturated solution of NaHCO3 and extracted with dichloromethane two times. The combined organic layer is successively washed with water (10 Vol x 2) and brine solution (5 Vol), dried over sodium sulphate, filtered and concentrated under reduced pressure. The crude product is purified by column chromatography.
Note:
- The most preferable solvent is DCM and the reaction is usually performed at 0 oC to -78 oC.
- DAST, (Diethylamino)sulfur trifluoride, is a nucleophilic fluorinating agent.
- DAST is heat sensitive and moisture sensitive. In general DAST reactions should not be heated.
- DAST is useful for dfluorination of carbonyl compounds as well
- Always look for safer alternatives to avoid DAST. There are several relatively safer reagents available to convert alcohol to fluoride
Safer alternatives to DAST:
- Deoxofluor — Relatively better
- Stable Fluorinating Reagent with Ease of Handling (FLUOLEAD®) – A safer substitute for DAST
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Fluorination of Alcohols Using DAST (TCI) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2016011390, page No. 382
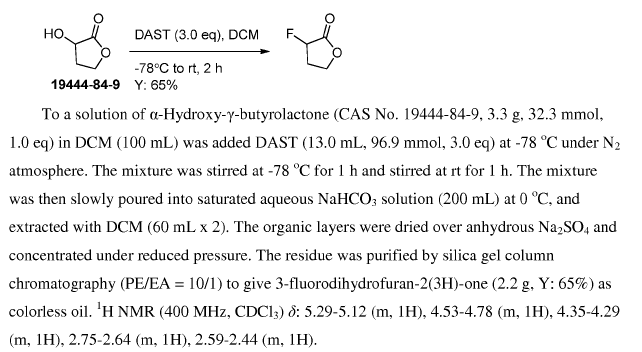
Green Chem.
It is not advisable to perform DAST reaction on large-scale.
Safer alternatives to DAST:
- Deoxofluor — Relatively better
- Stable Fluorinating Reagent with Ease of Handling (FLUOLEAD®) – A safer substitute for DAST
Scale-Up Typical Procedure:
- Development of a Kilogram-Scale Synthesis of cis-LC15-0133 Tartrate, a Potent Dipeptidyl Peptidase IV Inhibitor (OPRD, 2008) – 3.5 Kg batch; 4.5 Kg DAST is used
Green Chemistry Aspects:
DAST – References:
General Fluorination – References: