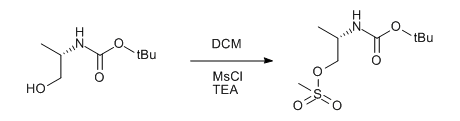
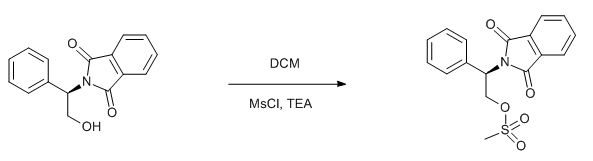
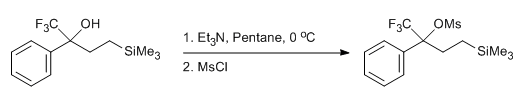
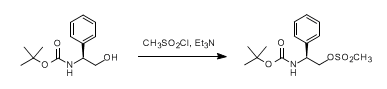
& Mechanism
Green Chem.
& Mechanism
Reaction & Reagents info
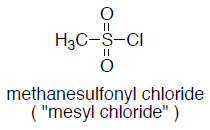
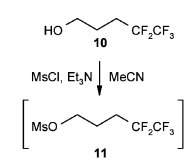
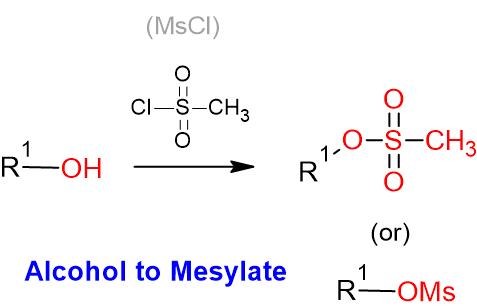
- OH– ion (hydroxide ion), as such, is a poor leaving group, as it is quite strong base. However, OH group shall be converted to a better leaving group such as OMs, OTs or OTf groups for further useful organic conversion
Sulfonates as better leaving groups
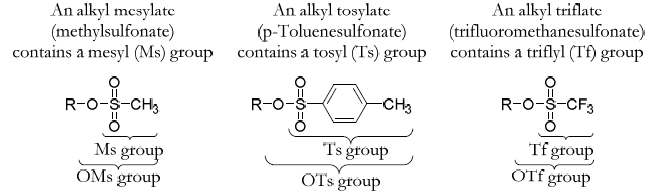
- Leaving group ability; OTf > OTs > OMs
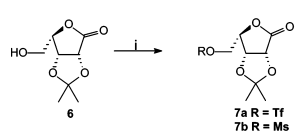
Other ways of synthesizing better leaving groups for SN2 reactions:
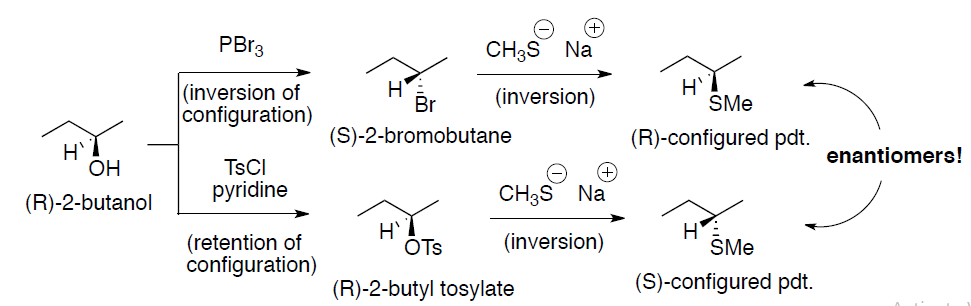
Stereochemical outcome of sulfonate ester (OMs, OTs and OTf):
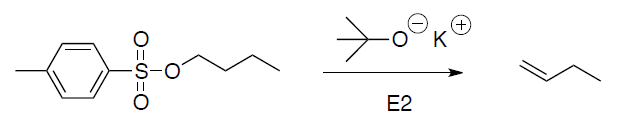
- Alkyl sulfonates undergo SN2 and E2 reaction in a manner identical to that of alkyl halides
(a) Substitution:
(b) Elimination:
For review papers and other articles,
refer to the tab "References"
Useful Links on Reagent & Reaction:
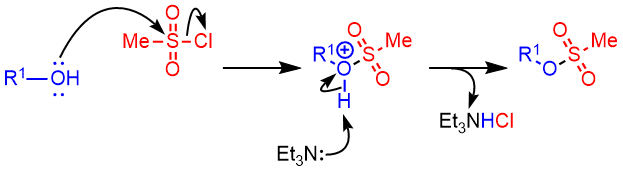
Mechanism
Alcohol to Mesylate – Mechanism
Additional details
General Procedure:
To a solution of alcohol (1 eq.) in dry DCM (10 Vol) at 0 oC was added triethylamine (1.5 eq.) followed by methanesulfonyl chloride (1.2 eq.) and stirred at 0 oC for 4 h (If the reaction does not proceed, it shall be brought to room temperature and stirred for 2 h). The reaction is monitored by TLC. After the completion of the reaction, the reaction mixture is diluted with water and layers separated. The aqueous layer is extracted with DCM. The organic layer is then successively washed with water (10 Vol x 2) and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure to get the desired compound.
Note:
- The preferable solvents are DCM, toluene
- Common bases are Pyridine, DIPEA, Et3N
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Mesylation of a Trifluoromethyl Carbinol (ChemSpider) — Open access
- Alcohol to Mesylate-1 (OrgSyn) — Open access
- Alcohol to Mesylate-2 (OrgSyn) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2007121484, page No. 90

WO2010045258, page No. 293

Green Chem.
Mesylates have been synthesized on large-scale and they are key intermediates in the manufacturing of pharmaceutical intermediates
Scale-Up Typical Procedure:
- Fulvestrant: From the Laboratory to Commercial-Scale Manufacture (OPRD,2010) – 119 g batch of 4,4,5,5,5-pentafluoropentanol; 87 Kg of Methanesulfonyl chloride is used
- A Concise, Economical, and Diastereoselective Synthesis of Methyl DGJ Isopropylidene: An Iminocyclitol Molecule Core for Analogue Synthesis (OPRD, 2006) – 50 g batch to synthesize mesylate; Triflate synthesis is also provided in the same article
Green Chemistry Aspects: