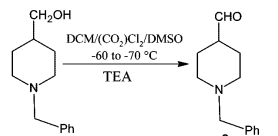
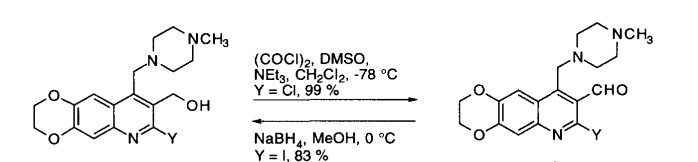
Reaction & Reagents info
- Swern Oxidation (DMSO, Oxalyl chloride) is a mild oxidizing system that converts primary alcohol to aldehyde and secondary alcohol to ketone
- It is one of the series of oxidations involving DMSO (refer to DMSO-activated oxidations)
Advantages
- Inexpensive oxidation method on manufacturing scale
- Also, it is less toxic, compared to chromium-based alternatives (PCC and PDC)
Disadvantages
- The liberation of gases viz., malodrous dimethylsulphide (Me2S) and poisonous carbon monoxide (CO) are to be handled appropriately
- It is important to maintain the reaction mixture at -78 oC. If the temperature is not maintained, there is a possibility of formation of mixed thioacetals (see mechanism)
Useful Links on Reagent & Reaction:
- DMSO –Oxalyl Chloride (Swern Oxidation) (Reagent Guide, ACS Green Chemistry Institute) – Green Chemistry info.
- Swern oxidation (SynArchive) – Excellent compilation of reaction schemes with references
Mechanism
General Oxidation Mechanism
DMSO, as such, is not a good oxidising agent. However, it needs to be activated by an activator [such as (COCl)2 in Swern Oxidation, DCC in Moffatt oxidation] so that DMSO shall act effective oxidising agent (refer to DMSO-activated Oxidations)
Activation of DMSO by Oxalyl Chloride
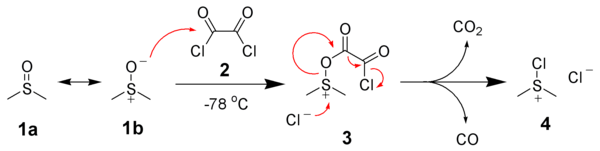
Swern Oxidation

Formation of side product in Swern Oxidation
In Swern oxidation employing (COCl)2, one mole each of CO, CO2 and Me2S (all gaseous products) are released. HCl gets converted to NEt3.HCl during the reaction
It is important to maintain the reaction mixture at -78 oC. If the temperature is not maintained, there is a possibility of formation of mixed thioacetals

Image from “chemistryworld.com”
Additional details
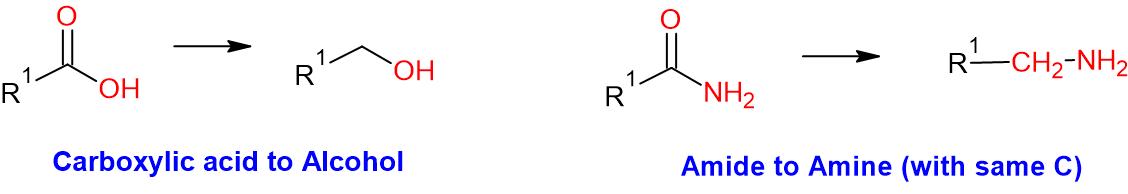
General Procedure:
To a solution of acid or amide (1 eq.) in dry THF (10 Vol) at 0 oC is added BH3.Me2S (1 eq.) dropwise for 1 h. It was brought to room temperature and stirred for 8 h. The reaction is monitored by TLC. (If the reaction does not proceed, it shall be heated to 40 to 50 oC) . After the completion of the reaction, the reaction mixture is cooled to 0 C and quenched by adding methanol or ethanol (Note: effervescence is observed). After stirring at room temperature for 2 h, the reaction mixture is poured into water (10 Vol) and extracted with DCM or EtOAc. The organic layer is then successively washed with water (10 Vol) and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure to get the desired compound. The crude product is purified by column chromatography.
Note:
- BH3.Me2S is one of the suitable reagents for the reduction of amides and acids. The reaction shall be done on large-scale. BH3.THF is also useful

Typical Procedure:
WO2010045258, page No. 127 (Acid to Alcohol)

Swern oxidation could be carried out on large-scale. However, the reaction involves the liberation of 1 eq. each of the gases such as Me2S (dimethylsulphide), CO (carbon monoxide), CO2. Appropriate safety controls are to be ensured while performing manufacturing. During work-up, HCl gets converted to amine salt (such as NEt3.HCl).
- Swern Oxidation is one of the inexpensive methods to manufacture aldehydes or ketones from Alcohols
- The liberation of gases viz., malodrous dimethylsulphide (Me2S), poisonous carbon monoxide (CO) and CO2 are to be handled appropriately
- It is important to maintain the reaction mixture at -78 oC. If the temperature is not maintained, there is a possibility of formation of mixed thioacetals (see mechanism in General Info section)
Scale-Up Typical Procedure:
- An Improved and Efficient Process for the Production of Donepezil Hydrochloride (OPRD, 2008) – 4 Kg batch; 3.5 Kg of DMSO & 3.7 Kg of (COCl)2 have been used
- Convergent Catalytic Asymmetric Synthesis of Camptothecin Analog GI147211C (Tetrahedron, 1997) – 100 g batch
Green Chemistry Aspects: