& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
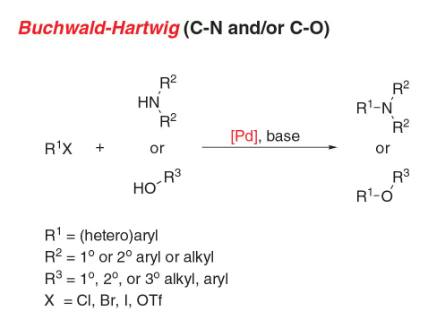
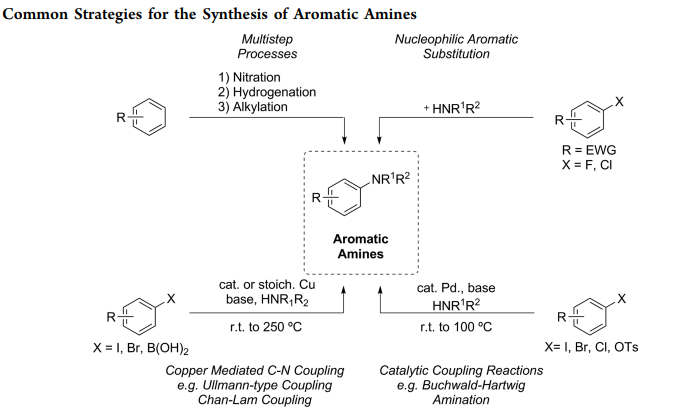
- Buchwald coupling: C-N bond formation involving amines and aryl halides
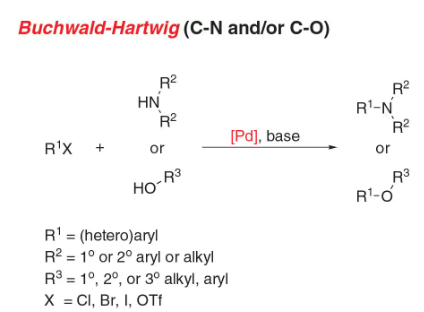
- Reactant-1 (Nucleophile): 1° or 2° Aliphatic/Aromatic Amine, or Imide, Amide, Sulfonamide, Sulfoximine
- Reactant-2 (Electrophile): Aryl Halide/Sulfonate
- Solvents: THF, Dioxane, Toluene
- Catalyst: Catalytic Palladium [Pd(PPh3)4 (tetrakis), (Ph3P)2PdCl2 (dikis)]
- Bases: NaOtBu, LiHMDS, K2CO3, Cs2CO3
Useful Links on Reagent & Reaction:
- Buchwald-Hartwig Coupling (SynArchive) – Excellent compilation of reaction schemes with references
- User’s Guide on Buchwald-Hartwig Coupling (Ref; D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50) – Amazing guide for C-N coupling (few summary images below)
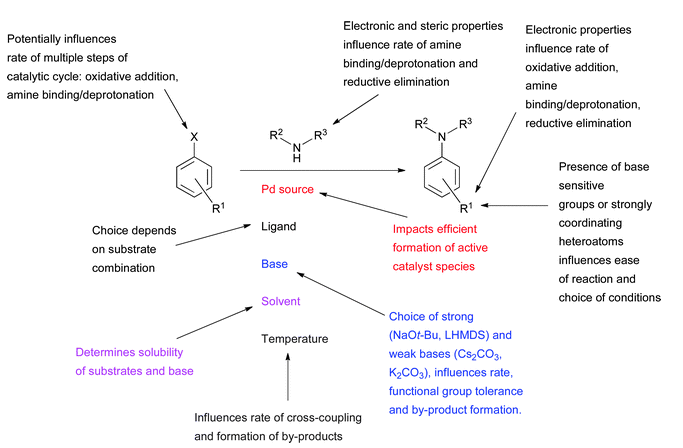
1. Factors influencing the outcome of a Pd-catalyzed amination reaction
Ref: D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50
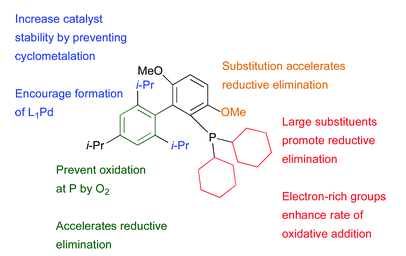
2. Important structural features of dialkylbiaryl phosphine ligands:
Ref: D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50
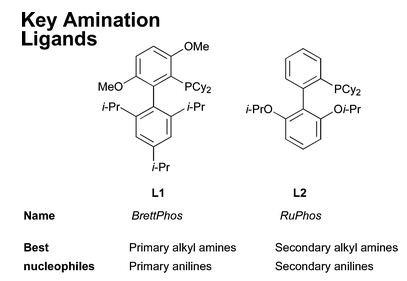
Important Dialkylbiaryl phosphine ligands for amination:
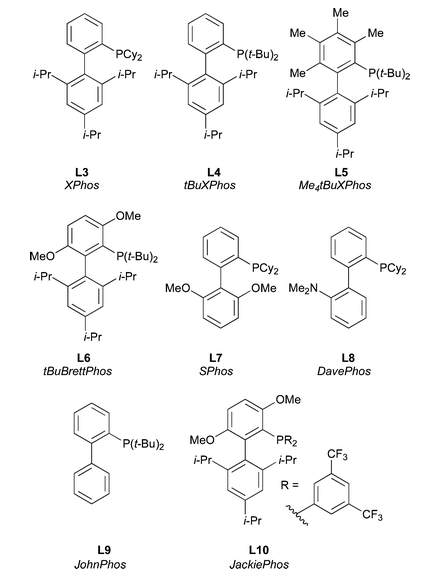
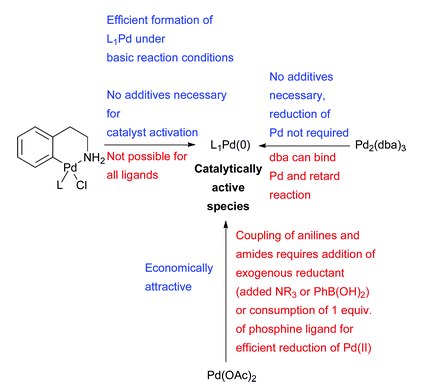
3. Considerations for choice of Pd source for amination reactions:
Ref: D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50
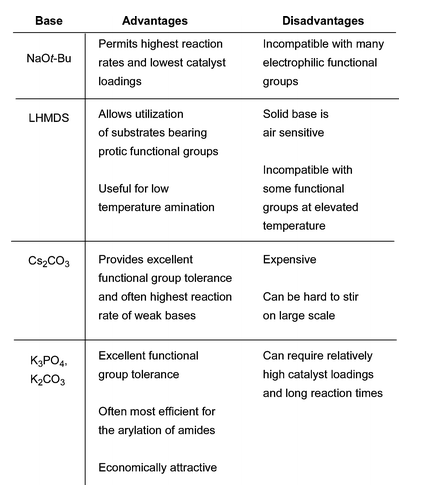
4. Comparison of bases typically used in Pd-catalyzed amination:
Ref: D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50
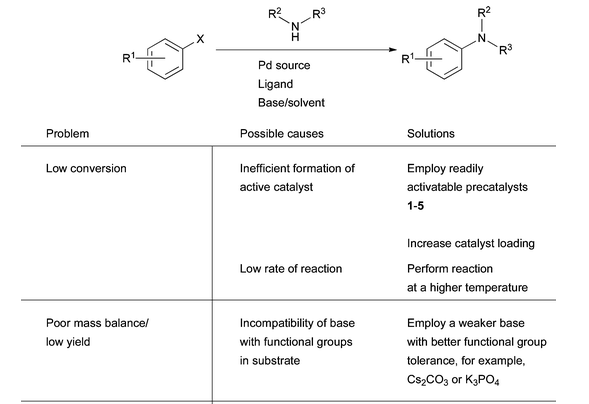
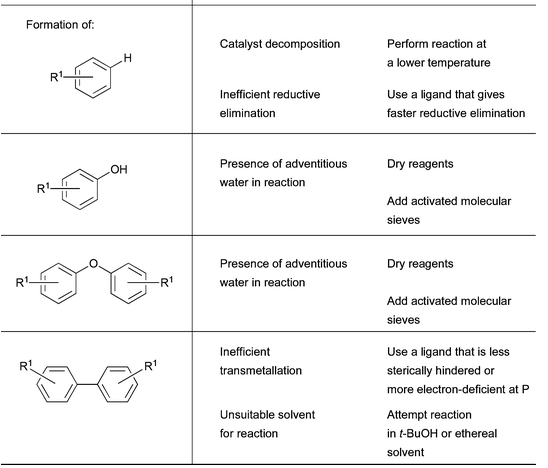
5. Simplistic troubleshooting guide Pd-catalyzed amination:
Ref: D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50
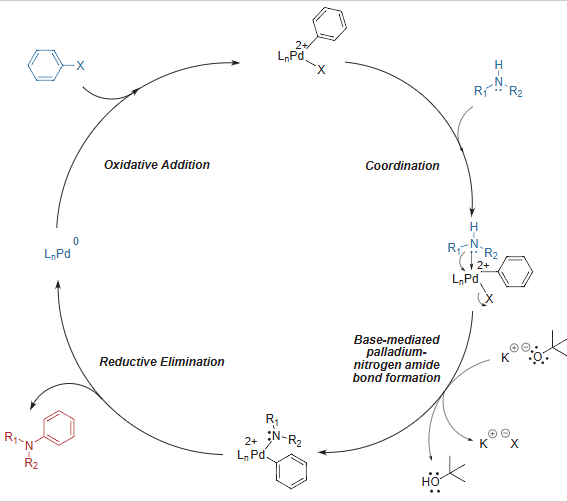
Mechanism
Buchwald-Hartwig Coupling – General Mechanism
- The mechanism follows Oxidative addition – Reductive elimination cycle.
Buchwald-Hartwig Coupling – Mechanism with specific example
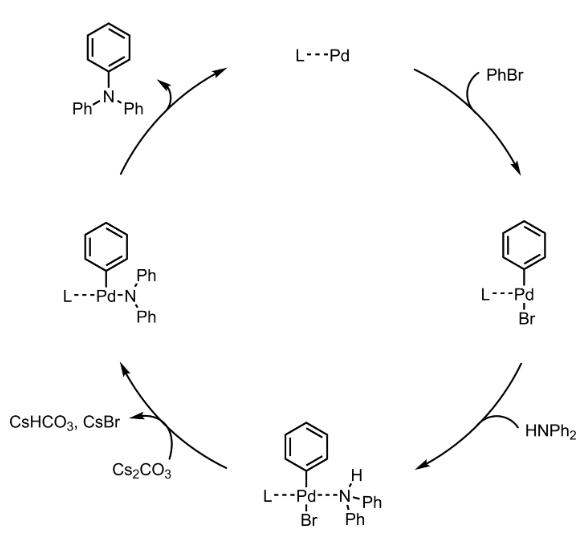
Additional details
General Procedure:
Bromo-aromatic ring (1 equiv.), aniline (1.5 equiv.), Cs2CO3 (10 equiv.), Pd(OAc)2 (0.05 equiv.), and BINAP (0.08 equiv.) were mixed in toluene (10 Vvol). The mixture was degassed, and stirred at 110 oC for 8 hours under nitrogen atmosphere. The resulting mixture was filtered with celite and the filtrate was concentrated. The resultant residue was purified by silica gel column to give rise to the desired compound.
Note:
- Better yields are obtained when the Pd (0) catalyst is complexed with chelating phosphine type ligands such as BINAP, DPPF, XANTPHOS, and DPBP or bidentate ligands (e.g: DBA)
- Bulky phosphines are required when aryl chloride is the substrate
- The base should be present in stoichiometric amounts
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Buchwald-Hartwig coupling of benzophenone imine with 4,7-dibromobenzo[c]-1,2,5-thiadiazole (ChemSpider) — Open access
- Buchwald-Hartwig amination of a bromopyridine with cyclohexane-1,2-diamine (ChemSpider) — Open access
- General protocol for Buchwald-Hartwig amination of aryl halides mediated by an in situ palladium/imidazolinium salt catalytic system (ChemSpider) — Open access
- Buchwald/Hartwig amination of bromopyridines (ChemSpider) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2007128568, page no. 56
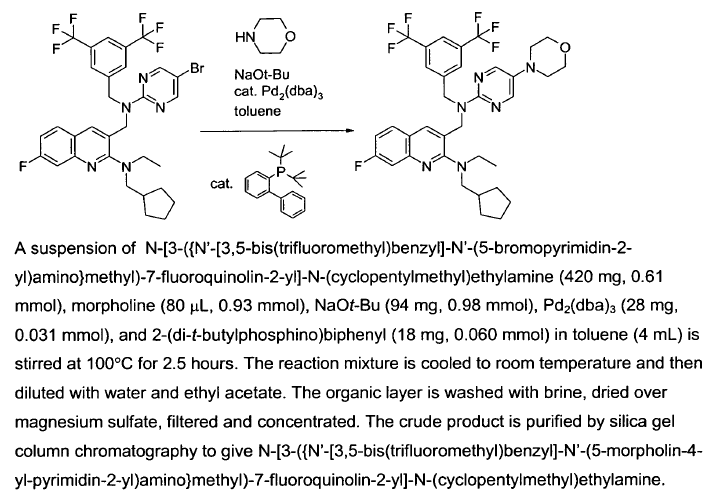
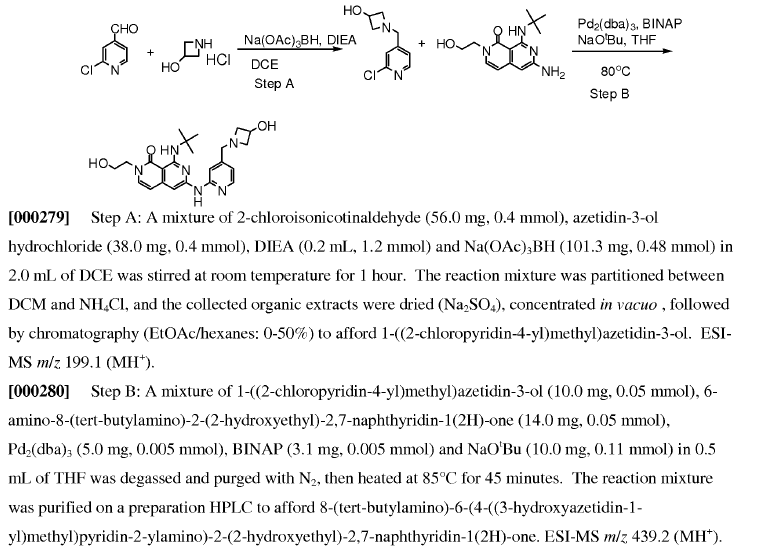
WO2011014515, page no. 110
- Step-A is Reductive amination using NaBH(OAc)3
- Step-B is Buchwald coupling
WO2007127635, page no. 245
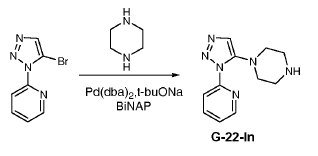
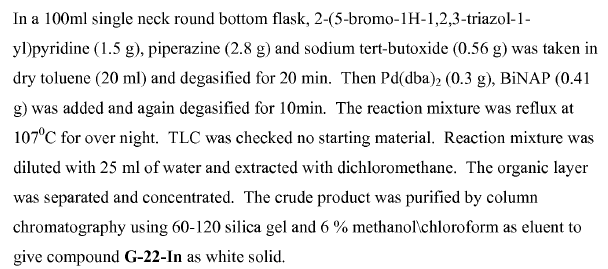
WO2011014515, page no. 111
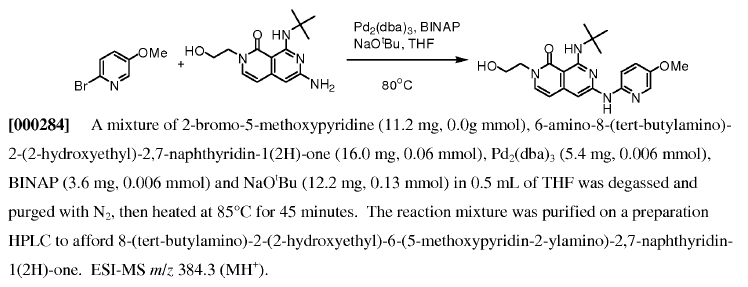
WO2011014515, page no. 108
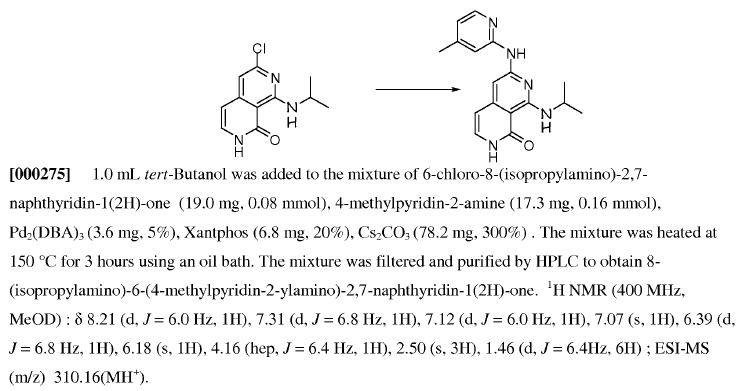
WO2007128568, pagen no. 53
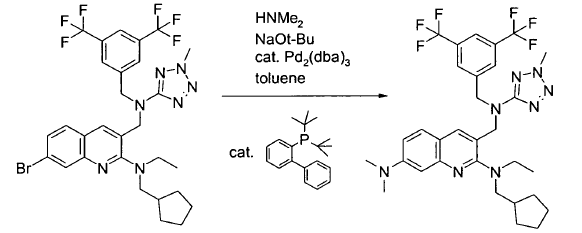
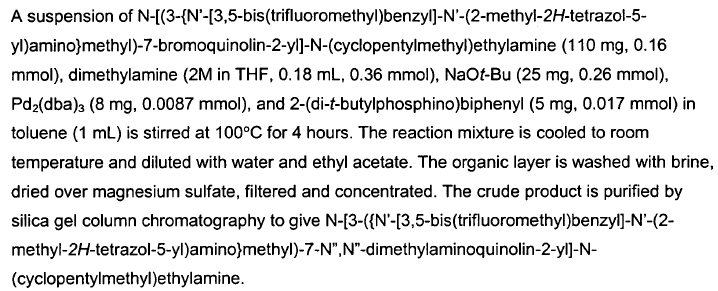
Green Chem
Buchwald-Hartwig coupling has been carried out on large-scale and there are several reports available in OPRD.
Scale-Up Typical Procedure:
- Kiloscale Buchwald–Hartwig Amination: Optimized Coupling of Base-Sensitive 6-Bromoisoquinoline-1-carbonitrile with (S)-3-Amino-2-methylpropan-1-ol (OPRD, 2014) – 2.5 Kg batch (Br derivative); A detailed account on Pd removal is reported
- Multi-Kilo Delivery of AMG 925 Featuring a Buchwald–Hartwig Amination and Processing with Insoluble Synthetic Intermediates (OPRD, 2015) – 7.3 Kg batch (amine derivative); 7.67 Kg (Cl derivative), Pd(OAc)2 (0.058 Kg) and BrettPhos (0.17 Kg) are used. Also, there is a procedure on the removal of Pd. are used.
Green Chemistry Aspects:
Buchwald-Hartwig Coupling – Reviews :
- The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 8, 1478–1483 – An excellent review article on Buchwald-Hartwig coupling






