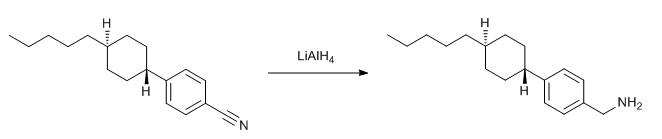
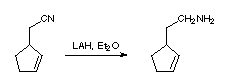& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
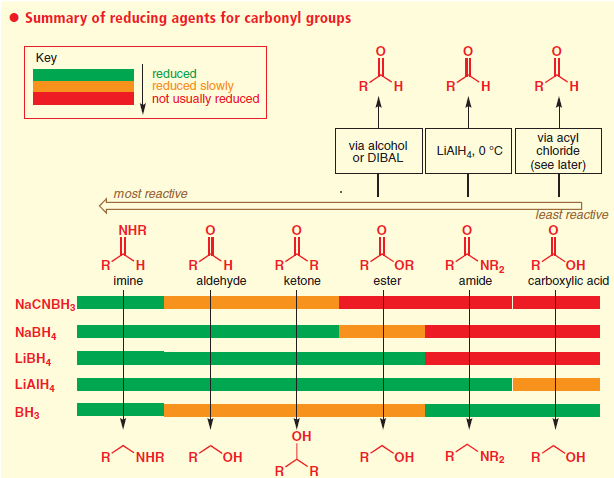
- LiAlH4 (Lithium Aluminium Hydride, LAH) is a very powerful reducing agent and reduces several functional groups (and hence not selective)
- LiAlH4 is highly flammable reagent and therefore it must be handled with care. It is not desirable to use LiAlH4 for large-scale. LAH should be avoided at best and be replaced with safer alternate reagents
- LiAlH4 (LAH) is available as powder or pellets
- LAH reactions are usually performed in ethereal solvents (e.g: THF) and it will be a suspension.
- LAH reacts violently with protic solvents such as alcohol, water (compare with NaBH4, wherein MeOH or EtOH is used as a reaction solvent)
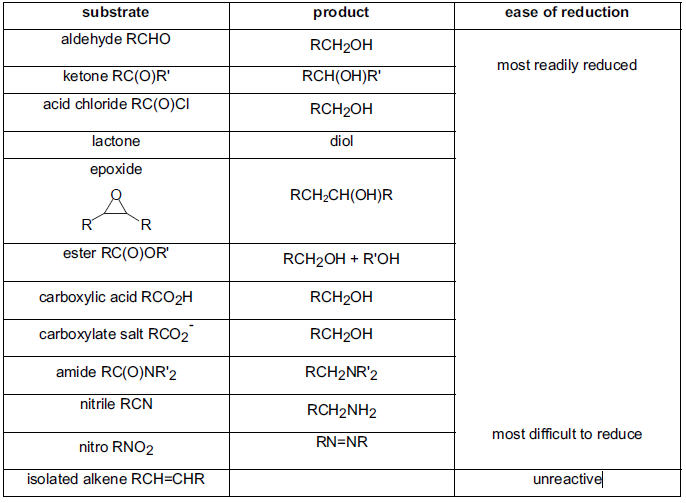
Ease of Reduction of some Functional Groups with LiAlH4:
- Borane is a strong Lewis acid that reacts readily with Lewis bases. Hence, Borane forms a stable complex with ethers such as THF or thioethers
Other reactions of LiAlH4 (LAH):
- Lactone can be reduced to Lactol using DIBAL-H
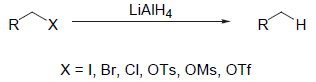
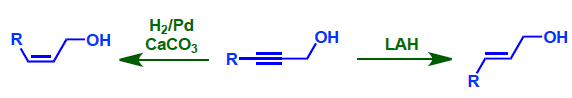
- LAH reduction is complementary to that of partial hydrogenation
Comparison of Reducing agents towards carbonyl compounds
Ref: Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford UP: Oxford, U.K., 2012; p 534.
Useful Links on Reagent & Reaction:
For review papers and other articles,
refer to the tab "References"
Mechanism
General Reduction Mechanism
Additional details

General Procedure:
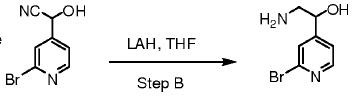
To a suspension of of Lithium aluminium hydride (LAH, 1.5 eq.) in THF (10 Vol) at 0 oC is added nitrile derivative (1 eq.) in THF and stirred at room temperature under nitrogen atmosphere for 4 h. The reaction is monitored by TLC. The reaction mixture is cooled to 0 oC and the unreacted LAH is quenched by adding successively water (1 Vol) , 10 % NaOH (1.5 Vol) , and finally again water (3 Vol). The resultant suspension is filtered through celite and washed with ethyl acetate/DCM. The layers are separated. The organic layer is then successively washed with water (10 Vol x 2) and brine solution, dried over sodium sulphate, filtered and concentrated under reduced pressure to get the desired compound. The crude product is purified by column chromatography.
When the desired compound is amine, acid-base work-up shall be included to remove nitrile from amine.
LAH shall be quenched by several methods
(a) by adding water (1 Vol) , 10 % NaOH (1.5 Vol), and finally again water (3 Vol).
(b) by adding saturated aqueous Na2SO4
(c) by adding aqueous solution of Rochelle’s salt (sodium potassium tartrate, KNaC4H4O6)
- Quenching reactions: Lithium Aluminium Hydride (LibreTexts Chemistry)
- Quenching reactions: Aluminium based reagents (LibreTexts Chemistry) – LAH & DIBAL-H
Note:
- LAH is not a suitable reagent for large-scale. Even for small scale, it is preferable to use alternate reagents that are relatively safe to handle, as compared to LAH
- LAH reduces (a) acid and esters to alcohols, (b) nitriles and amides to amine
- To reduce nitrile to amine, in place of LAH, other conditions such as (a) Raney Ni, H2 or (b) Pd/C, H2 shall also be used
- LAH, being a strong reducing agent, reduces several functional groups, making it least preferable.
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Reduction of a benzonitrile (ChemSpider) — Open access
- Reduction of a nitrile (ChemSpider) — Open access
For more details on large-scale reactions and OPRD procedures,
refer to the tab "Scale-up & Green Chem"
WO2011014515, page No. 113

Green Chem
LiAlH4 is highly flammable reagent and therefore it must be handled with care. It is not desirable to use LiAlH4 for large-scale. LAH should be avoided at best and be replaced with safer alternate reagents
Green Chemistry Aspects: