& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
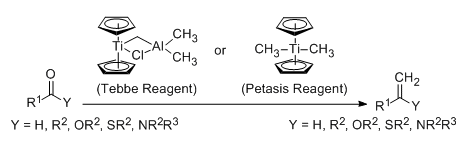
- Tebbe and Petasis reagents are methylenation reagents of carbonyl compounds such as Aldehydes, Ketones, Esters, Lactones, thioesters and amides
Tebbe reagent:
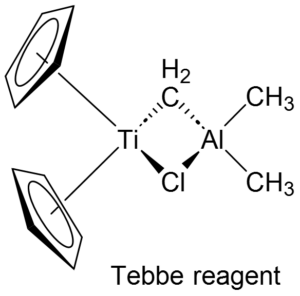
- Tebbe reagent is a red solid that is pyrophoric (ignites spontaneously in air) and typically used under nitrogen or argon. It is commercially available as a solution in toluene
- The Ti and Al bonds are bridged by both CH2 and chloride ligands
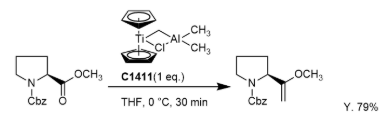
- Tebbe reagent reacts readily with ketones, aldehydes and amides, but its reactivity with esters is very slow. Therefore, if the compound contains ketone and ester, ketone group reacts selectively with one equivalents of Tebbe reagent
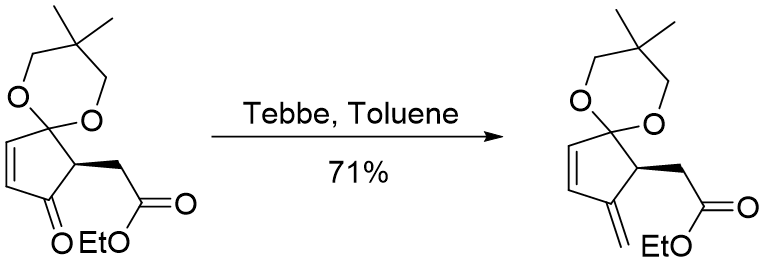
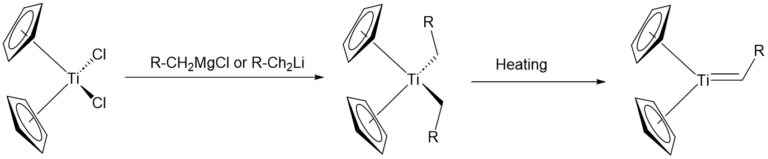
Petasis reagent:
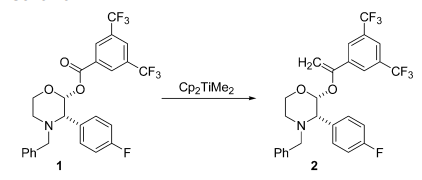
- Petasis reagent is more stable towards moisture and air, as compared to Tebbe reagent.
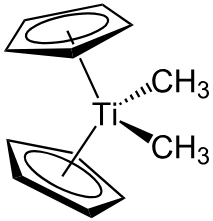
Tebbe reagent Vs Petasis reagent:
- Preparation of Tebbe reagent: Though it is commercially available, it shall be synthesized as well. It is usually prepared by treating titanocene dichloride with two equivalents of trimethylaluminum in toluene
- Preparation of Petasis reagent: It is prepared by treating titanocenedichloride with methyl magnesium chloride or methyllithium (for methylenation). Homologs of Petasis reagent aresynthesized by using the corresponding alkyllithium reagents.
- Both form titanocene methylidene (Schrock carbene) as the key intermediate
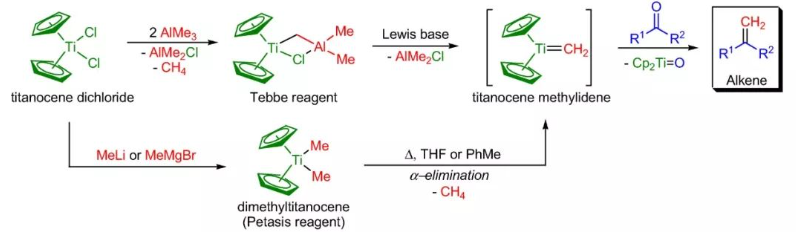
- Both Tebbe reagent and Petasis reagent are useful for methylenation
- Tebbe reagent works well only for methylenation (i.e conversion of -CO to -C=CH2) of various carbonyl compounds. However, other alkenyl groups can not be introduced.
- Unlike Tebbe reagent, Petasis reagent is used to introduce other alkenyl groups as well
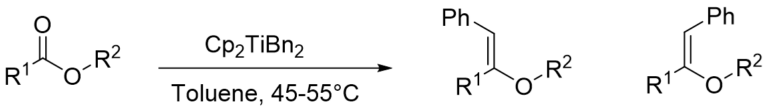
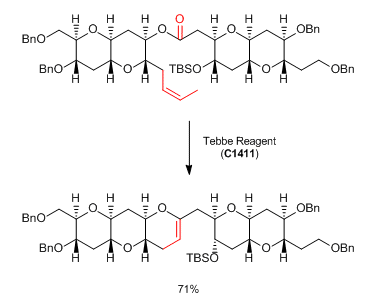
Reactions involving similar chemistry:
Tebbe and Petasis reagents in Ring-Closing Metathesis (RCM):
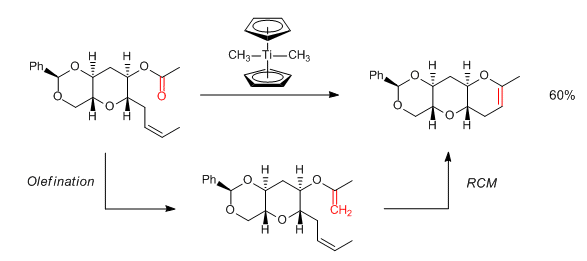
Useful Links on Tebbe and Petasis reagents:
- Tebbe-Petasis Olefination (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
Tebbe and Petasis reaction – Mechanism
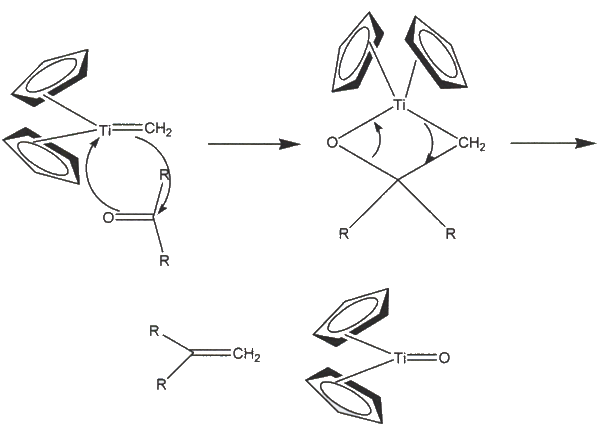
- Both Tebbe and Petasis reagents form titanocene methylidene (Schrock carbene) as the key intermediate
- The carbene reacts with the carbonyl compound to result in an oxatitanacyclobutane intermediate, which then breaks down to form an alkene
Additional details
Tebbe and Petasis Olefinations:

General Procedure-1:
To a solution of aldehyde or ketone (1.0 eq) in THF (10 Vol) at 0 °C is added a solution of the Tebbe reagent (0.5 M in toluene, 3.0 eq). The reaction is brought to room temperature and stirred for 2 h. The reaction is monitored by TLC. The reaction mixture is diluted with MTBE and then quenched with aqueous NaOH. The resultant mixture is extracted with DCM or EtOAc (10 Vol) two times. The combined organic layer is washed with water and brine solution (5 Vol), dried over Na2SO4 and concentrated to afford the crude compound. The crude material is purified by column chromatography.
Note:
- The Tebbe reagent is commercially available.
- It can be prepared by treating titanocene dichloride with two equivalents of trimethylaluminum in toluene. This generates a methylene-bridged titanium-aluminum complex (also called Tebbe reagent).
Typical Procedure:
- Methylenation Reagent (TCI) — Please click the section ” Applications & Literature” — Open access
Patent references
Green Chem
Tebbe and Petasis olefinations have been performed on large-scale. There are few OPRD articles available
Scale-Up Typical Procedure:
- Dimethyltitanocene: From Millimole to Kilomole (OPRD, 2004) – 250 Kg batch (ester derivative)
Green Chemistry Aspects: