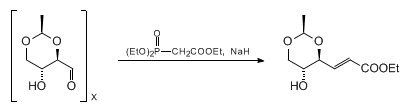
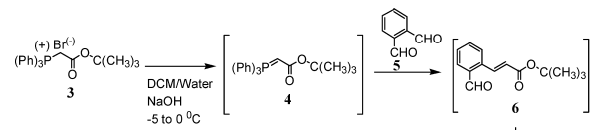
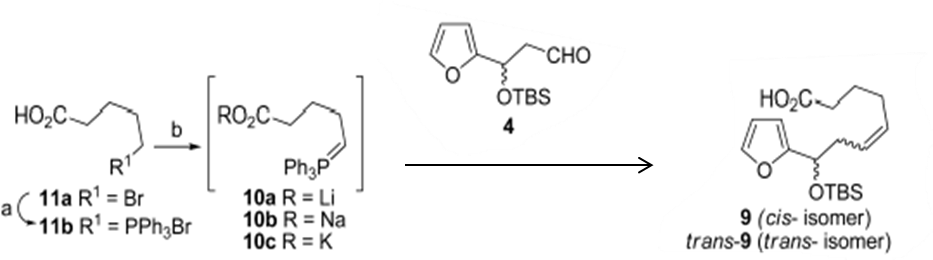
& Mechanism
Green Chem
& Mechanism
Reaction & Reagents info
- Wittig reaction is helpful in synthesizing alkenes from aldehydes/ketones and phosphorus ylides
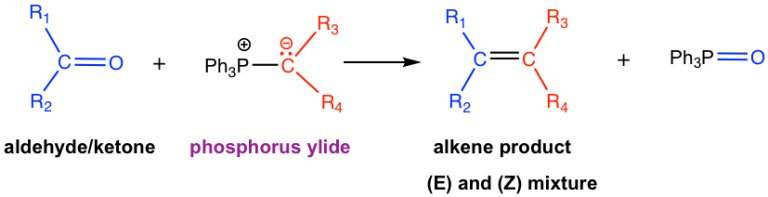
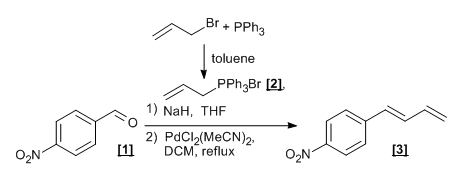
- The phosphorus ylide is prepared from 1° or 2° alkyl halide, PPh3 and a base, as depicted below
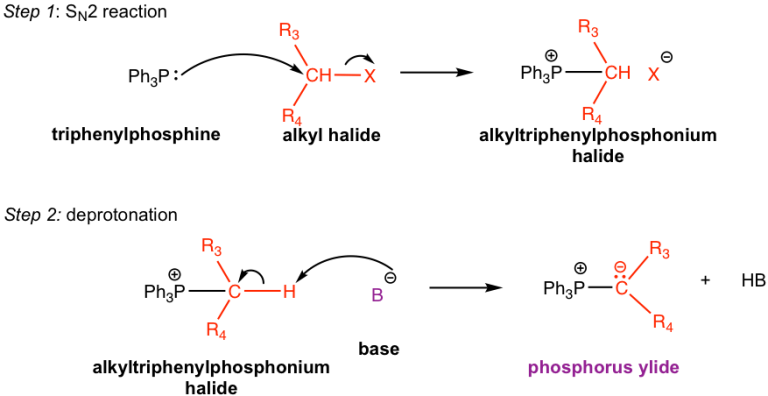
Resonance structures of Phosphorus Ylides:

- Reactant-1: Aldehyde or Ketone
- Reactant-2 (Wittig reagent): Phosphorus Ylide, prepared from 1° or 2° alkyl halide ((Cl, Br, I) or OTs
- Reagents: Trialkyl or triaryl phsphines (e.g: PPh3)
- Bases: K2CO3, Cs2CO3, KtOBu, NaH, n-BuLi – Depending on the nature of the substrate
- Solvents: THF,
- Reaction type: Nucleophilic Addition of Phosphorus Ylides
- Products: Olefin
- Bond formation: C=C
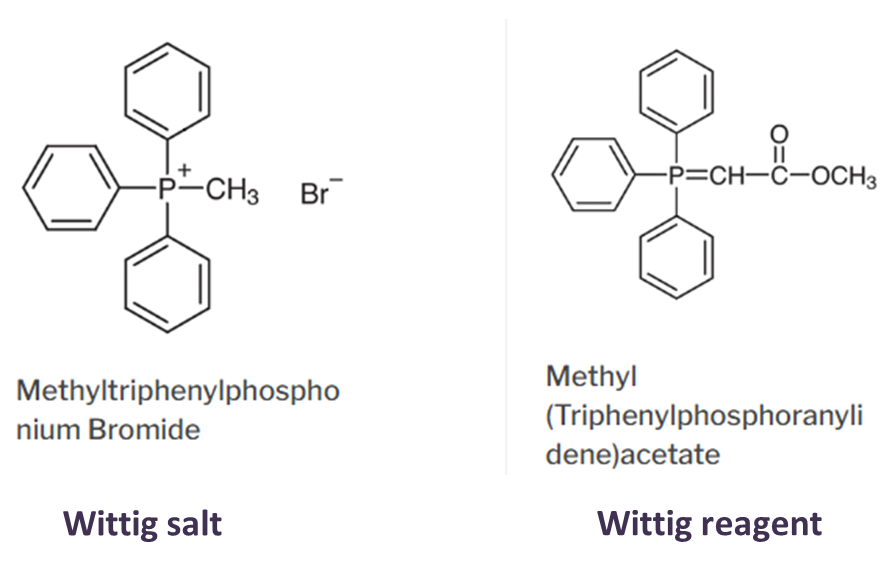
Wittig salt is Phosphonium salt and Wittig reagent is Phosphonium Ylide
Stereochemistry
- Z-Alkenes: Stabilized ylides [having electron withdrawing group (EWG) to stabilize the negative charge on the carbon] result in Z-alkenes
- E-Alkenes: Non-stabilized ylides gives rise to E-alkenes
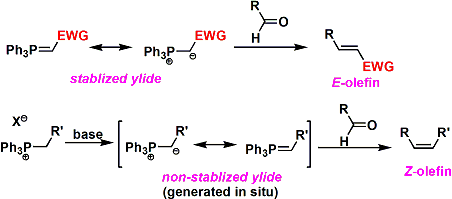
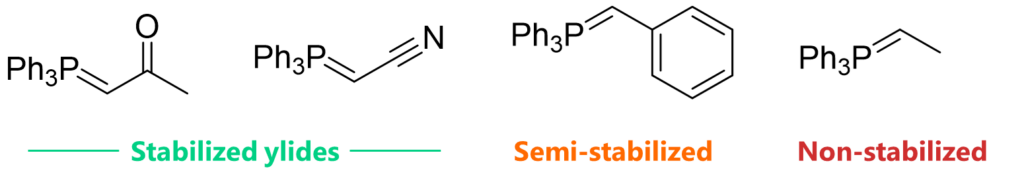
Stabilized and Non-stabbilized Ylides
- Ylides shall be classified into two categories: Stabilized Ylides & Non-stabilized Ylides (The stability indicates the ability of the substituent to stabilize the negative charge on carbanion)
- Stabilized Ylides: When the substituent attached to carbanion is EWG, Ylide gets stabilized through resonance. EWG such as esters, nitriles, sulfone stabilizes the ylide, making it less reactive but more selective
- Stabilized Ylides lead to thermodynamically stable E-alkene. These reactions shall be performed at high temp.
- Non-stabilized Ylides: When the substituent is alkyl group (EDG), there is no stabilization of carbanion and hence Ylides are not stabilized, making it more reactive
- Non-stabilized Ylides lead to kinetically favored Z-alkene. These reactions are performed at low temp.
- Semi-stabilized Ylides: When the substituent is aryl or alkenyl, it falls between stabilized and non-stabilized. Here, the stereoselectivity is poor, leading to mixture of both Z- and E-alkenes
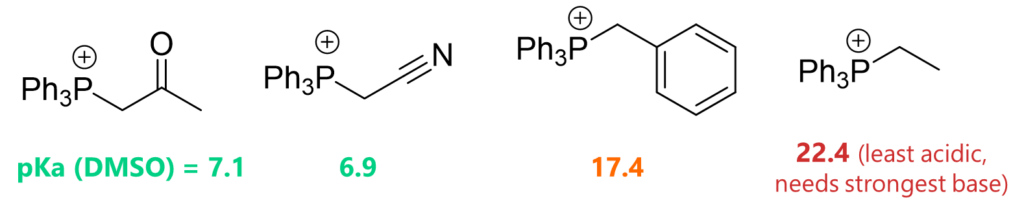
- Also, stabilized ylides have acidic proton (i.e. -CH next to EWG is more acidic) and hence milder bases are sufficient enough to deprotonate the carbon.
Schlosser Modification of Wittig reaction
Useful Links on Reagent & Reaction:
- Wittig reaction (SynArchive) – Excellent compilation of reaction schemes with references
For review papers and other articles,
refer to the tab "References"
Mechanism
Wittig reaction – Mechanism
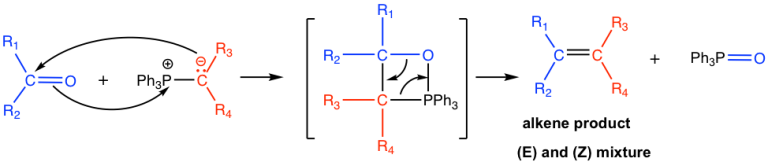
The mechanism involves (a) Nucleophilic addition of Ylide onto carbonyl group via [2+2] cycloaddition, leading to betaine intermediate (oxaphosphetane ring) which decides the stereochemical outcome (b) ring opening results in alkene. The driving force for the ring opening is the formation phosphine oxide
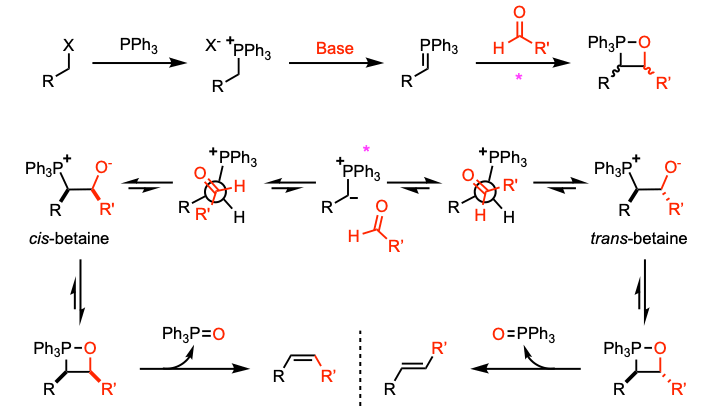
Wittig reaction – Stereoselectivity
- The orientation of substituents on the oxaphosphetane ring (Betaine) dictates the stereoselecivity. In the oxaphosphetane ring, there are two chiral centers and hence cis- and trans- diastereomers are formed.
- cis- leads to Z-alkenes and trans- leads to E-alkenes
Additional details
Wittig reaction:

General Procedure-1 (Wittig salt):
To a solution of Wittig salt and KOtBu in dry THF at 0 oC is added Aldehyde/Ketone and stirred for 4 h at the same temp. The reaction is monitored by TLC. After the completion of the reaction, it is brought to room temperature. The reaction mixture is quenched by adding aq. NH4Cl or aq. 1N HCl (10 Vol) and stirred for 2 h. The resultant mixture is extracted with DCM or EtOAc (10 Vol) two times. The combined organic layer is washed with water and brine solution (5 Vol), dried over Na2SO4 and concentrated to afford the crude compound. The crude material is purified by column chromatography.
General Procedure-2 (Wittig salt):
To a solution of Wittig salt in dry THF at 0 oC, n-BuLi is added and stirred at the same temperature for 2 h. The reaction mixture is cooled to -78 oC. Aldehyde/Ketone is added dropwise at -78 oC and the reaction mixture is stirred at the same temperature for 2 h and then at 0 oC for 1h. The reaction is monitored by TLC. The reaction mixture is quenched by adding aq. NH4Cl or aq. 1N HCl (10 Vol) at 0 oC and stirred for 2 h. The resultant mixture is extracted with DCM or EtOAc (10 Vol) two times. The combined organic layer is washed with water and brine solution (5 Vol), dried over Na2SO4 and concentrated to afford the crude compound. The crude material is purified by column chromatography.
General Procedure-3 (with stabilized ylide) :
To a solution of Aldehyde/Ketone (1 equiv) in dry THF (15 mL) is added Wittig reagent (2 equiv) at RT. The reaction mixture was stirred and heated to 50 °C for 2 h. The solvent is then removed under reduced pressure and the crude material is purified by column chromatography.
- Wittig salt is Phosphonium salt and Wittig reagent is Phosphonium Ylide
- Some of the stabilized phosphonium ylides (Wittig reagents) are available commercially. When they are involved in the reaction, it shall be used as such. As the Ylide is also ready, the base is not used here. For stabilized ylides, the reaction mixture shall be heated
- Stabilized Ylides: When the substituent attached to carbanion is EWG, Ylide gets stabilized through resonance. EWG such as esters, nitriles, sulfone stabilizes the ylide, making it less reactive but more selective
- Stabilized Ylides lead to thermodynamically stable E-alkene. These reactions shall be performed at high temp.
Note:
- In Wittig reaction, unstabilised Wittig salt (e.g Ph3PCH3I) is used. It often results in Z-alkenes.
- On the contrary, in Wittig-Horner reaction (also called Horner-Wadsworth-Emmons reaction), stabilized Wittig Ylide (e.g: phosphonate carbanions) are used. This leads to E-alkenes predominantly
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
Typical Procedure:
- Wittig Reaction (ChemSpider) — Open access
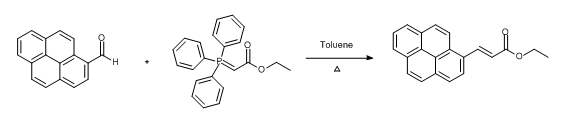
- Wittig reaction of aldehydes and subsequent Pd-catalysed diene isomerisation (ChemSpider) — Open access
- Wittig reaction-1 (OrgSyn) — Open access
- Wittig reaction-2 (OrgSyn) — Open access
- Wittig reaction-3 (OrgSyn) — Open access
For more details on reactions and reagents,
refer to the tab "Reaction, Reagents and Mechanism"
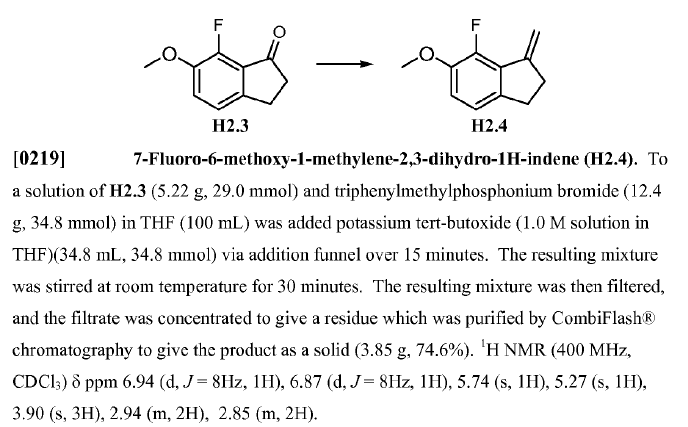
WO2010045258, page No. 82
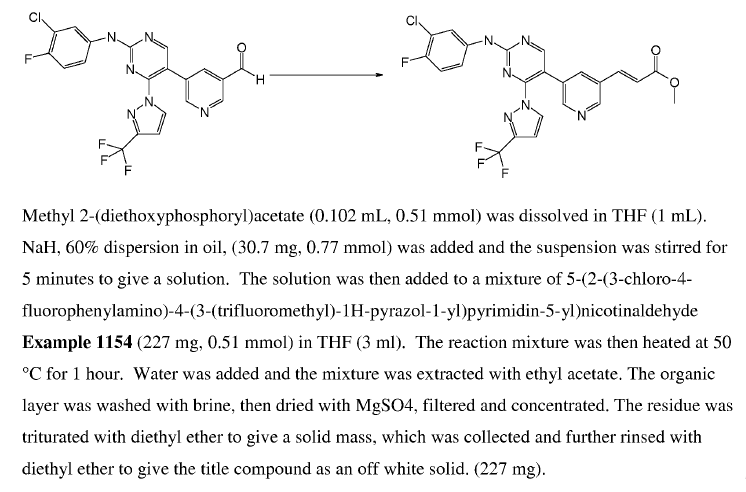
WO2010038081, page No. 878
Green Chem
Wittig reaction has been carried out on large-scale and there are several reports available in OPRD
Scale-Up Typical Procedure:
- A Practical and Scalable Method for Manufacturing JAK Inhibitor ASP3627 (OPRD, 2019) – In this article, 6 steps have been performed in a telescopic manner, among which Wittig reaction is one of steps. 69 Kg batch (Nitrile derivative) gets converted to aldehyde, which undergoes Wittig reaction; 74.6 Kg of Wittig salt, viz., (methoxymethyl)-triphenylphosphonium chloride and 137.5 Kg NaHMDS (S (38.7% in THF) are used.
- A Facile, One-Pot Synthesis of Lacidipine Using in Situ Generation of Wittig Intermediates (OPRD, 2009) – 2.05 Kg batch (aldehyde derivative) and 5 Kg Wittig salt are used
Role of TPPA in Wittig reaction:
- The Manufacture of a Homochiral 4-Silyloxycyclopentenone Intermediate for the Synthesis of Prostaglandin Analogues (OPRD, 2012) – 0.4 Kg batch (aldehyde derivative), 0.9 Kg Wittig reagent and 0.96 Kg TPPA are used
- Tripyrrolidinophosphoric acid triamide (TPPA) is a Lewis basic additive that can be used as a replacement for HMPA (hexamethylphosphoramide) in Wittig reactions, especially those involving organolithium compounds.
- TPPA can improve the stereoselectivity of Wittig reactions, potentially leading to increased yields of E-alkenes.
- TPPA can help to stabilize the phosphonium ylide, which can then react more effectively with the carbonyl compound
Green Chemistry Aspects:
- Solvent-Free Wittig Reaction: A Green Organic Chemistry Laboratory Experiment (Org. Process Res. Dev. 2012, 16, 12, 1905–1916)
Useful articles for Scale-up:
Wittig reaction – Reviews :
- The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 4, 863–927